Hip Osteoarthritis and the Risk of Lacunar Stroke: A Two-Sample Mendelian Randomization Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
2.2. Data Sources
2.3. Selection of the Genetic Instrumental Variables (IVs)
2.4. Statistical Analyses
3. Results
3.1. The Final Instruments in the MR Analyses
3.2. Association between Hip OA and Risk of Lacunar Stroke
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, S.; Wu, S.; Zhang, S.; Wu, B. Advances in Understanding the Pathogenesis of Lacunar Stroke: From Pathology and Pathophysiology to Neuroimaging. Cerebrovasc. Dis. 2021, 50, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Egeto, P.; Fischer, C.E.; Ismail, Z.; Smith, E.E.; Schweizer, T.A. Lacunar stroke, deep white matter disease and depression: A meta-analysis. Int. Psychogeriatr. 2014, 26, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Griebe, M.; Fischer, E.; Kablau, M.; Eisele, P.; Wolf, M.E.; Chatzikonstantinou, A.; Gass, A.; Hennerici, M.G.; Szabo, K. Thrombolysis in patients with lacunar stroke is safe: An observational study. J. Neurol. 2014, 261, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Gore, M.; Bansal, K.; Asuncion, R.M.D. Lacunar Stroke. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Vermeer, S.E.; Longstreth, W.T., Jr.; Koudstaal, P.J. Silent brain infarcts: A systematic review. Lancet Neurol. 2007, 6, 611–619. [Google Scholar] [CrossRef]
- Regenhardt, R.W.; Das, A.S.; Lo, E.H.; Caplan, L.R. Advances in Understanding the Pathophysiology of Lacunar Stroke: A Review. JAMA Neurol. 2018, 75, 1273–1281. [Google Scholar] [CrossRef]
- Wiseman, S.; Marlborough, F.; Doubal, F.; Webb, D.J.; Wardlaw, J. Blood markers of coagulation, fibrinolysis, endothelial dysfunction and inflammation in lacunar stroke versus non-lacunar stroke and non-stroke: Systematic review and meta-analysis. Cerebrovasc. Dis. 2014, 37, 64–75. [Google Scholar] [CrossRef]
- Shi, Y.; Wardlaw, J.M. Update on cerebral small vessel disease: A dynamic whole-brain disease. Stroke Vasc. Neurol. 2016, 1, 83–92. [Google Scholar] [CrossRef]
- Kwan, A.; Wei, J.; Dowling, N.M.; Power, M.C.; Nadareishvili, Z. Cognitive Impairment after Lacunar Stroke and the Risk of Recurrent Stroke and Death. Cerebrovasc. Dis. 2021, 50, 383–389. [Google Scholar] [CrossRef]
- Das, A.S.; Regenhardt, R.W.; Feske, S.K.; Gurol, M.E. Treatment Approaches to Lacunar Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 2055–2078. [Google Scholar] [CrossRef]
- Xia, B.; Di, C.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Murphy, N.J.; Eyles, J.P.; Hunter, D.J. Hip Osteoarthritis: Etiopathogenesis and Implications for Management. Adv. Ther. 2016, 33, 1921–1946. [Google Scholar] [PubMed]
- Courties, A.; Berenbaum, F. Is hip osteoarthritis preventable? Jt. Bone Spine 2020, 87, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Cawley, D.T.; Guerin, S.J.; Walsh, J.; Simpkin, A.; Masterson, E.L. The significance of hand dominance in hip osteoarthritis. Semin. Arthritis Rheum. 2015, 44, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; van der Esch, M.; Hinman, R.S.; Peat, G.; de Zwart, A.; Quicke, J.G.; Runhaar, J.; Knoop, J.; van der Leeden, M.; de Rooij, M.; et al. How does hip osteoarthritis differ from knee osteoarthritis? Osteoarthr. Cartil. 2022, 30, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Tanislav, C.; Kostev, K. Osteoarthritis and incidence of stroke and transient ischemic attack in 320,136 adults followed in general practices in the United Kingdom. Jt. Bone Spine 2021, 88, 105104. [Google Scholar] [CrossRef]
- Swain, S.; Coupland, C.; Mallen, C.; Kuo, C.F.; Sarmanova, A.; Bierma-Zeinstra, S.M.A.; Englund, M.; Prieto-Alhambra, D.; Doherty, M.; Zhang, W. Temporal relationship between osteoarthritis and comorbidities: A combined case control and cohort study in the UK primary care setting. Rheumatology 2021, 60, 4327–4339. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, J.; Ju, L.; Sun, L.; Tse, L.A.; Kinra, S.; Li, Y. Osteoarthritis & stroke: A bidirectional mendelian randomization study. Osteoarthr. Cartil. 2022, S1063-4584, 00770–00771. [Google Scholar]
- Zuber, V.; Colijn, J.M.; Klaver, C.; Burgess, S. Selecting likely causal risk factors from high-throughput experiments using multivariable Mendelian randomization. Nat. Commun. 2020, 11, 29. [Google Scholar] [CrossRef]
- Tachmazidou, I.; Hatzikotoulas, K.; Southam, L.; Esparza-Gordillo, J.; Haberland, V.; Zheng, J.; Johnson, T.; Koprulu, M.; Zengini, E.; Steinberg, J.; et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat. Genet. 2019, 51, 230–236. [Google Scholar] [CrossRef]
- Traylor, M.; Persyn, E.; Tomppo, L.; Klasson, S.; Abedi, V.; Bakker, M.K.; Torres, N.; Li, L.; Bell, S.; Rutten-Jacobs, L.; et al. Genetic basis of lacunar stroke: A pooled analysis of individual patient data and genome-wide association studies. Lancet Neurol. 2021, 20, 351–361. [Google Scholar] [CrossRef]
- Liu, X.; Peng, Y.; Tao, R.; Meng, L.; Li, X. Mendelian Randomization Study of Causal Relationship between Omega-3 Fatty Acids and Risk of Lung Cancer. Biomed Res. Int. 2022, 2022, 2786567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, W.; Sun, W.; Wang, X.; Tian, M.; Pei, L.L.; Liu, K.; Liang, J.; Zhou, L.; Lu, J.; et al. Heart Failure and Ischemic Stroke: A Bidirectional and Multivariable Mendelian Randomization Study. Front. Genet. 2021, 12, 771044. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLlife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.J.; Spiller, W.; Jung, K.J.; Lee, J.Y.; Kimm, H.; Back, J.H.; Lee, S.; Jee, S.H. Causal Associations Between Serum Bilirubin Levels and Decreased Stroke Risk: A Two-Sample Mendelian Randomization Study. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.F.; Minelli, C.; Sheehan, N.A.; Thompson, J.R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 2015, 34, 2926–2940. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Zhang, Z.; Liu, D.; Ji, L.; Huang, S.; Cao, L.; Wei, N.; Ye, D.; Ma, Y.; Lian, X. Genetic predisposition to Parkinson’s disease and risk of cardio and cerebrovascular disease: A Mendelian randomization study. Parkinsonism Relat. Disord. 2022, 94, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, L.; Liu, W.; Tian, M.; Wang, X.; Liang, J.; Wang, Y.; Ding, L.; Pei, L.; Lu, J.; et al. Stroke and Myocardial Infarction: A Bidirectional Mendelian Randomization Study. Int. J. Gen. Med. 2021, 14, 9537–9545. [Google Scholar] [CrossRef]
- Lynch, T.S.; O’Connor, M.; Minkara, A.A.; Westermann, R.W.; Rosneck, J.T. Biomarkers for Femoroacetabular Impingement and Hip Osteoarthritis: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2019, 47, 2242–2250. [Google Scholar] [CrossRef]
- Chamorro, A.; Revilla, M.; Obach, V.; Vargas, M.; Planas, A.M. The-174G/C polymorphism of the interleukin 6 gene is a hallmark of lacunar stroke and not other ischemic stroke phenotypes. Cerebrovasc. Dis. 2005, 19, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Boehme, A.K.; McClure, L.A.; Zhang, Y.; Luna, J.M.; Del Brutto, O.H.; Benavente, O.R.; Elkind, M.S. Inflammatory Markers and Outcomes After Lacunar Stroke: Levels of Inflammatory Markers in Treatment of Stroke Study. Stroke 2016, 47, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Lespasio, M.J.; Sultan, A.A.; Piuzzi, N.S.; Khlopas, A.; Husni, M.E.; Muschler, G.F.; Mont, M.A. Hip Osteoarthritis: A Primer. Perm. J. 2018, 22, 17–084. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Rysinska, A.; Garland, A.; Rolfson, O.; Aspberg, S.; Eisler, T.; Garellick, G.; Stark, A.; Hailer, N.P.; Sköldenberg, O. Increased Long-Term Cardiovascular Risk After Total Hip Arthroplasty: A Nationwide Cohort Study. Medicine 2016, 95, e2662. [Google Scholar] [CrossRef]
- Hawker, G.A.; Croxford, R.; Bierman, A.S.; Harvey, P.J.; Ravi, B.; Stanaitis, I.; Lipscombe, L.L. All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: A population based cohort study. PLoS ONE 2014, 9, e91286. [Google Scholar]
- Hawker, G.A.; Croxford, R.; Bierman, A.S.; Harvey, P.; Ravi, B.; Kendzerska, T.; Stanaitis, I.; King, L.K.; Lipscombe, L. Osteoarthritis-related difficulty walking and risk for diabetes complications. Osteoarthr. Cartil. 2017, 25, 67–75. [Google Scholar] [CrossRef]
- Mast, H.; Thompson, J.L.; Lee, S.H.; Mohr, J.P.; Sacco, R.L. Hypertension and diabetes mellitus as determinants of multiple lacunar infarcts. Stroke 1995, 26, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Martí-Vilalta, J.L. Lacunar stroke. Expert Rev. Neurother. 2009, 9, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Laita, L.; Estébanez-de-Miguel, E.; Martín-Nieto, G.; Bueno-Gracia, E.; Fortún-Agúd, M.; Jiménez-Del-Barrio, S. Effects of non-pharmacological conservative treatment on pain, range of motion and physical function in patients with mild to moderate hip osteoarthritis. A systematic review. Complement Ther. Med. 2019, 42, 214–222. [Google Scholar] [CrossRef]
- Chung, K.M.; Ho, C.H.; Chen, Y.C.; Hsu, C.C.; Chiu, C.C.; Lin, H.J.; Wang, J.J.; Huang, C.C. Chronic Pain Increases the Risk for Major Adverse Cardiac and Cerebrovascular Events: A Nationwide Population-Based Study in Asia. Pain Med. 2020, 21, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Brotman, D.J.; Golden, S.H.; Wittstein, I.S. The cardiovascular toll of stress. Lancet 2007, 370, 1089–1100. [Google Scholar] [CrossRef]
- McBeth, J.; Chiu, Y.H.; Silman, A.J.; Ray, D.; Morriss, R.; Dickens, C.; Gupta, A.; Macfarlane, G.J. Hypothalamic-pituitary-adrenal stress axis function and the relationship with chronic widespread pain and its antecedents. Arthritis Res. Ther. 2005, 7, R992–R1000. [Google Scholar] [CrossRef]
- Parish, S.; Arnold, M.; Clarke, R.; Du, H.; Wan, E.; Kurmi, O.; Chen, Y.; Guo, Y.; Bian, Z.; Collins, R.; et al. Assessment of the Role of Carotid Atherosclerosis in the Association Between Major Cardiovascular Risk Factors and Ischemic Stroke Subtypes. JAMA Netw. Open. 2019, 2, e194873. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef] [Green Version]




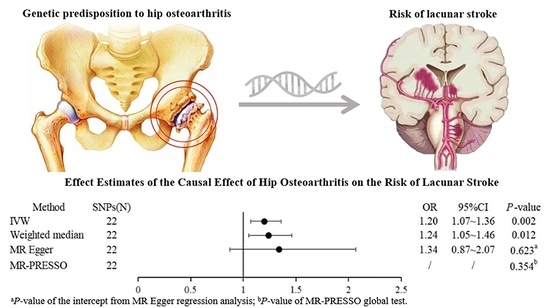
| Method | SNPs (N) | OR | 95%CI | MR p-Value | Heterogeneity Q/p-Value | Pleiotropy Intercept p-Value |
|---|---|---|---|---|---|---|
| IVW | 22 | 1.20 | 1.07~1.36 | 0.002 | 23.35/0.325 | |
| Weighted median | 22 | 1.24 | 1.05~1.46 | 0.012 | ||
| MR Egger | 22 | 1.34 | 0.87–2.07 | 0.203 | 0.623 a | |
| MR-PRESSO | 22 | / | / | 0.354 b |
| SNP | Chr | Position | Effect Allele | SNPs-Hip Osteoarthritis | SNPs-Lacunar Stroke | ||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | p-Value | β | SE | p-Value | ||||
| rs10492367 | 12 | 28014970 | T | 0.15 | 0.01 | 1.25 × 10−24 | −0.02 | 0.03 | 0.51 |
| rs10896015 | 11 | 65323725 | A | −0.08 | 0.01 | 2.74 × 10−9 | 0.01 | 0.02 | 0.56 |
| rs11059094 | 12 | 122606837 | T | 0.08 | 0.01 | 7.38 × 10−11 | −0.02 | 0.02 | 0.41 |
| rs115740542 | 6 | 26123502 | C | 0.13 | 0.02 | 1.60 × 10−8 | 0.06 | 0.04 | 0.12 |
| rs11583641 | 1 | 183906245 | T | −0.08 | 0.01 | 5.57 × 10−10 | −0.04 | 0.02 | 0.07 |
| rs12040949 | 1 | 150447462 | T | −0.07 | 0.01 | 2.83 × 10−8 | −0.02 | 0.02 | 0.43 |
| rs12209223 | 6 | 76164589 | A | 0.16 | 0.02 | 3.88 × 10−16 | 0.04 | 0.03 | 0.31 |
| rs13300602 | 9 | 129412938 | G | 0.07 | 0.01 | 1.65 × 10−9 | 0.03 | 0.02 | 0.23 |
| rs1835323 | 2 | 43512130 | T | −0.07 | 0.01 | 4.56 × 10−8 | −0.02 | 0.02 | 0.37 |
| rs1913707 | 4 | 13039440 | G | −0.08 | 0.01 | 2.96 × 10−11 | 0.01 | 0.02 | 0.64 |
| rs2396502 | 6 | 45357699 | C | 0.08 | 0.01 | 2.12 × 10−12 | 0.00 | 0.02 | 0.88 |
| rs2785988 | 1 | 219744138 | A | 0.08 | 0.01 | 7.30 × 10−11 | 0.00 | 0.02 | 0.83 |
| rs2836618 | 21 | 40048295 | A | 0.09 | 0.01 | 3.20 × 10−11 | 0.05 | 0.02 | 0.03 |
| rs3774355 | 3 | 52817778 | A | 0.09 | 0.01 | 8.20 × 10−14 | 0.02 | 0.02 | 0.23 |
| rs4252548 | 19 | 55879672 | T | 0.28 | 0.04 | 1.96 × 10−12 | 0.11 | 0.08 | 0.15 |
| rs4338381 | 1 | 103572927 | G | −0.10 | 0.01 | 4.37 × 10−15 | −0.01 | 0.02 | 0.70 |
| rs62063281 | 17 | 44038785 | G | 0.10 | 0.01 | 5.30 × 10−12 | 0.07 | 0.03 | 0.01 |
| rs74767794 | 1 | 184006128 | G | −0.08 | 0.01 | 2.56 × 10−9 | 0.02 | 0.02 | 0.47 |
| rs7571789 | 2 | 70714793 | C | −0.09 | 0.01 | 3.26 × 10−14 | −0.05 | 0.02 | 0.03 |
| rs79056043 | 12 | 59289598 | G | 0.16 | 0.03 | 1.33 × 10−9 | 0.03 | 0.05 | 0.46 |
| rs798748 | 4 | 1716770 | C | 0.07 | 0.01 | 2.50 × 10−9 | 0.00 | 0.02 | 0.83 |
| rs80287694 | 6 | 55636940 | G | 0.11 | 0.02 | 2.66 × 10−9 | 0.05 | 0.03 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Li, F.; Cao, L.; Wang, Y.; Xiao, J.; Zhou, X.; Tian, T. Hip Osteoarthritis and the Risk of Lacunar Stroke: A Two-Sample Mendelian Randomization Study. Genes 2022, 13, 1584. https://doi.org/10.3390/genes13091584
Shen Y, Li F, Cao L, Wang Y, Xiao J, Zhou X, Tian T. Hip Osteoarthritis and the Risk of Lacunar Stroke: A Two-Sample Mendelian Randomization Study. Genes. 2022; 13(9):1584. https://doi.org/10.3390/genes13091584
Chicago/Turabian StyleShen, Yi, Fuju Li, Lina Cao, Yunyun Wang, Jing Xiao, Xiaoyi Zhou, and Tian Tian. 2022. "Hip Osteoarthritis and the Risk of Lacunar Stroke: A Two-Sample Mendelian Randomization Study" Genes 13, no. 9: 1584. https://doi.org/10.3390/genes13091584