A Review on CYP11A1, CYP17A1, and CYP19A1 Polymorphism Studies: Candidate Susceptibility Genes for Polycystic Ovary Syndrome (PCOS) and Infertility
Abstract
:1. Introduction
2. Biochemical Pathways in PCOS
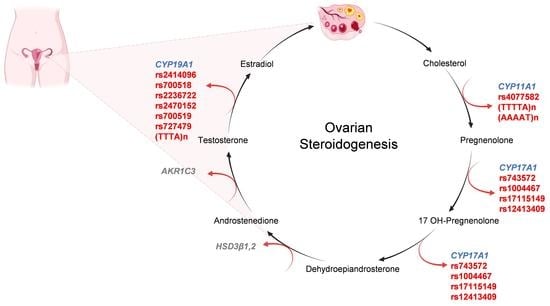
3. Ovarian Steroidogenesis
4. CYP Genes and PCOS with Infertility
4.1. CYP11A1 Gene
4.2. CYP17A1 Gene
4.3. CYP19A1 Gene
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADIPOQ | Adiponectin |
| ARI | Aldehyde reductase 1 |
| AMH | Anti-Mullerian hormone |
| AIs | Aromatase inhibitors |
| CYP21 | 21-hydroxylase gene |
| CYP11A | Cholesterol side-chain cleavage enzyme |
| CYP19A1 | Cytochrome P450 family 19 subfamilies A member 1 |
| CYPs | Cytochrome P450 enzymes |
| CNS | Central nervous systems |
| DHEA | Dehydroepiandrosterone |
| FSH | Follicle-stimulating hormone |
| GWAS | Genome-wide association study |
| HA | Hyperandrogenism |
| HCG | Human choriogonadotropin |
| HSDs | Hydroxysteroid dehydrogenases |
| INSR | Insulin receptor |
| INS | Insulin |
| IRS-1 | Insulin receptor substrate 1 |
| IVF | In vitro fertilization |
| LHCGR | Luteinizing hormone/Choriogonadotropin receptor |
| LH | luteinizing hormone |
| MMP | Matrix metalloproteinase |
| PCOS | Polycystic ovary syndrome |
| SNPs | Single nucleotide polymorphisms |
| StAR | Steroidogenic acute regulatory protein |
| T2DM | Type 2 diabetes mellitus |
| UTR | Untranslated region |
| SHBG | Sex hormone-binding globulin |
References
- Visser, J.A. The importance of metabolic dysfunction in polycystic ovary syndrome. Nat. Rev. Endocrinol. 2021, 17, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Qin, P.; Yin, J.; Shi, Y.; Xuan, Y.; Chen, Z.; Zhou, X.; Yu, H.; Peng, D.; Wang, B. Clinical manifestations of polycystic ovary syndrome and associations with the vaginal microbiome: A cross-sectional based exploratory study. Front. Endocrinol. 2021, 12, 662725. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Dunaif, A. Diagnosis of polycystic ovary syndrome. Endocrinol. Metab. Clin. N. Am. 2021, 50, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Gambineri, A.; Cecchetti, C.; Altieri, P.; Ribichini, D.; Lo Preiato, V.; Fanelli, F.; Pagotto, U. Secondary PCOS: Well-defined causes, leading to the PCOS phenotype. In Polycystic Ovary Syndrome; Elsevier: Amsterdam, The Netherlands, 2022; pp. 15–22. [Google Scholar] [CrossRef]
- Geraghty, P. The Interaction of Menopause and Chronic Disease. In Each Woman’s Menopause An Evidence Based Resource; Springer: Berlin/Heidelberg, Germany, 2022; pp. 91–120. [Google Scholar] [CrossRef]
- Walker, K.; Decherney, A.H.; Saunders, R. Menstrual dysfunction in PCOS. Clin. Obstet. Gynecol. 2021, 64, 119–125. [Google Scholar] [CrossRef]
- Tehrani, F.R.; Behboudi-Gandevani, S.; Yarandi, R.B.; Naz, M.S.G.; Carmina, E. Prevalence of acne vulgaris among women with polycystic ovary syndrome: A systemic review and meta-analysis. Gynecol. Endocrinol. 2021, 37, 392–405. [Google Scholar] [CrossRef]
- Almeshari, W.K.; Alsubaie, A.K.; Alanazi, R.I.; Almalki, Y.A.; Masud, N.; Mahmoud, S.H. Depressive and anxiety symptom assessment in adults with polycystic ovarian syndrome. Depression Res. Treat. 2021, 2021, 6652133. [Google Scholar] [CrossRef]
- Pundir, C.S.; Deswal, R.; Narwal, V.; Dang, A. The prevalence of polycystic ovary syndrome: A brief systematic review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [CrossRef]
- Guo, F.; Huang, Y.; Fernando, T.; Shi, Y. Altered Molecular Pathways and Biomarkers of Endometrial Receptivity in Infertile Women with Polycystic Ovary Syndrome. Reprod. Sci. 2022, 1, 1–11. [Google Scholar] [CrossRef]
- Chen, X.; Koivuaho, E.; Piltonen, T.T.; Gissler, M.; Lavebratt, C. Reply: Association of Maternal Polycystic Ovary Syndrome or Anovulatory Infertility with Obesity and Diabetes in Offspring: A Population-Based Cohort Study. Hum. Reprod. 2021, 37, 193–194. [Google Scholar] [CrossRef]
- Kałużna, M.; Człapka-Matyasik, M.; Wachowiak-Ochmańska, K.; Moczko, J.; Kaczmarek, J.; Janicki, A.; Piątek, K.; Ruchała, M.; Ziemnicka, K. Effect of central obesity and hyperandrogenism on selected inflammatory markers in patients with PCOS: A WHtR-matched case-control study. J. Clin. Med. 2020, 9, 3024. [Google Scholar] [CrossRef]
- Firoozabadi, A.D.; Firouzabadi, R.D.; Eftekhar, M.; Bafghi, A.S.T.; Shamsi, F. Maternal and neonatal outcomes among pregnant women with different polycystic ovary syndrome phenotypes: A cross-sectional study. Int. J. Reprod. Biomed. 2020, 18, 339. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mo, M.; Xiao, S.; Li, L.; Hu, X.; Hong, L.; Wang, L.; Lian, R.; Huang, C.; Zeng, Y.; et al. Pregnancy outcomes of women with polycystic ovary syndrome for the first in vitro fertilization treatment: A retrospective cohort study with 7678 patients. Front. Endocrinol. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Deng, Q. Epigenetic inheritance of polycystic ovary syndrome—Challenges and opportunities for treatment. Nat. Rev. Endocrinol. 2021, 17, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Padmanabhan, V.; Walters, K.A.; Campbell, R.E.; Benrick, A.; Giacobini, P.; Dumesic, D.A.; Abbott, D.H. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr. Rev. 2020, 41, bnaa010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murrin, C.M.; Kelly, G.E.; Tremblay, R.; Kelleher, C.C. Body mass index and height over three generations: Evidence from the Lifeways cross-generational cohort study. BMC Public Health 2012, 12, 81. [Google Scholar] [CrossRef]
- Van Dijk, S.J.; EpiSCOPE, M.O.; Molloy, P.; Varinli, H.; Morrison, J.; Muhlhausler, B.S. Epigenetics and human obesity. Int. J. Obes. 2015, 39, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [Green Version]
- Skinner, M.K. Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol. Cell. Endocrinol. 2014, 398, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Abbott, D.H.; Dumesic, D.A.; Levine, J.E. Hyperandrogenic origins of polycystic ovary syndrome—Implications for pathophysiology and therapy. Expert Rev. Endocrinol. Metab. 2019, 14, 131–143. [Google Scholar] [CrossRef]
- Mimouni, N.E.H.; Paiva, I.; Barbotin, A.-L.; Timzoura, F.E.; Plassard, D.; Le Gras, S.; Ternier, G.; Pigny, P.; Catteau-Jonard, S.; Simon, V.; et al. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021, 33, 513–530. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Hoyos, L.R.; Chazenbalk, G.D.; Naik, R.; Padmanabhan, V.; Abbott, D.H. Mechanisms of intergenerational transmission of polycystic ovary syndrome. Reproduction 2020, 159, R1–R13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeger, K.; Saben, J.L.; Moley, K.H. Transmission of metabolic dysfunction across generations. Physiology 2017, 32, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Li, J.; Su, S.; Cao, Y.; Wang, Z.; Zhao, S.; Zhao, H. PCOS-GWAS Susceptibility Variants in THADA, INSR, TOX3, and DENND1A are associated with metabolic syndrome or insulin resistance in women with PCOS. Front. Endocrinol. 2020, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, H.; Chen, Z.-J. Genome-wide association studies for polycystic ovary syndrome. Semin. Reprod. Med. 2016, 34, 224–229. [Google Scholar] [CrossRef]
- Hiam, D.; Moreno-Asso, A.; Teede, H.J.; Laven, J.S.; Stepto, N.K.; Moran, L.J.; Gibson-Helm, M. The genetics of polycystic ovary syndrome: An overview of candidate gene systematic reviews and genome-wide association studies. J. Clin. Med. 2019, 8, 1606. [Google Scholar] [CrossRef] [Green Version]
- Teede, H.J.; Tay, C.T.; Joham, A.E. Polycystic ovary syndrome: An intrinsic risk factor for diabetes compounded by obesity. Fertil. Steril. 2021, 115, 1449–1450. [Google Scholar] [CrossRef]
- Jiang, N.-X.; Li, X.-L. The disorders of endometrial receptivity in PCOS and its mechanisms. Reprod. Sci. 2021, 1–12. [Google Scholar] [CrossRef]
- Kara, M.; Ozcan, S.S.; Aran, T.; Kara, O.; Yilmaz, N. Evaluation of endometrial receptivity by measuring HOXA-10, HOXA-11, and leukemia inhibitory factor expression in patients with polycystic ovary syndrome. Gynecol. Minim. Invasive Ther. 2019, 8, 118–122. [Google Scholar] [CrossRef]
- Abutorabi, E.S.; Rashidi, B.H.; Irani, S.; Haghollahi, F.; Bagheri, M. Investigation of the FSHR, CYP11, and INSR mutations and polymorphisms in iranian infertile women with Polycystic Ovary Syndrome (PCOS). Rep. Biochem. Mol. Biol. 2021, 9, 470–477. [Google Scholar] [CrossRef]
- Baldi, M.; Caserta, D. The genetics of polycystic ovary syndrome. Reprod. Biomed. Online 2006, 13, 10. [Google Scholar] [CrossRef]
- Chaudhary, H.; Patel, J.; Jain, N.K.; Joshi, R. The role of polymorphism in various potential genes on polycystic ovary syndrome susceptibility and pathogenesis. J. Ovarian Res. 2021, 14, 1–21. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, T.; Sudhir, N.; Kaur, A. Association Analysis of CYP11A1 variants with polycystic ovary syndrome: A case-control study from North India. Reprod. Sci. 2021, 28, 2951–2960. [Google Scholar] [CrossRef]
- Zeng, X.; Xie, Y.-J.; Liu, Y.-T.; Long, S.-L.; Mo, Z.-C. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin. Chim. Acta 2020, 502, 214–221. [Google Scholar] [CrossRef]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Massaro, M.G.; Morgante, G.; Petraglia, F. Genetic, hormonal and metabolic aspects of PCOS: An update. Reprod. Biol. Endocrinol. 2016, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- McGee, E.A. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Otto-Buczkowska, E.; Gliwice, M.S.C.I.; Grzyb, K.; Jainta, N. Polycystic ovary syndrome (PCOS) and the accompanying disorders of glucose homeostasis among girls at the time of puberty. Pediatr. Endocrinol. Diabetes Metab. 2018, 24, 40–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yang, M.; Luan, P.; Jia, W.; Liu, Q.; Ma, Z.; Dang, J.; Lu, H.; Ma, Q.; Wang, Y.; et al. Associations between cytochrome P450 (CYP) gene single-nucleotide polymorphisms and second-to-fourth digit ratio in chinese university students. Med Sci. Monit. 2021, 27, e930591-1. [Google Scholar] [CrossRef]
- Di Nardo, G.; Zhang, C.; Marcelli, A.G.; Gilardi, G. Molecular and structural evolution of cytochrome P450 aromatase. Int. J. Mol. Sci. 2021, 22, 631. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Liu, F.; Wu, J.; Xia, Y.; Zhao, Z.; Zhao, Y.; Ren, H.; Kong, X. Clinical and genetic characteristics of 17 α-Hydroxylase/17, 20-Lyase Deficiency: C.985_987delTACinsAA mutation of CYP17A1 prevalent in the chinese han population. Endocr. Pract. 2021, 27, 137–145. [Google Scholar] [CrossRef]
- Wang, M.; Strand, M.J.; Lanser, B.J.; Santos, C.; Bendelja, K.; Fish, J.; Esterl, E.A.; Ashino, S.; Abbott, J.K.; Knight, V.; et al. Expression and activation of the steroidogenic enzyme CYP11A1 is associated with IL-13 production in T cells from peanut allergic children. PLoS ONE 2020, 15, e0233563. [Google Scholar] [CrossRef]
- Goldstone, J.; Sundaramoorthy, M.; Zhao, B.; Waterman, M.R.; Stegeman, J.J.; Lamb, D.C. Genetic and structural analyses of cytochrome P450 hydroxylases in sex hormone biosynthesis: Sequential origin and subsequent coevolution. Mol. Phylogenet. Evol. 2016, 94, 676–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharani, N.; Waterworth, D.M.; Batty, S.; White, D.; Gilling-Smith, C.; Conway, G.S.; McCarthy, M.; Franks, S.; Williamson, R. Association of the steroid synthesis gene Cyp11a with polycystic ovary syndrome and hyperandrogenism. Hum. Mol. Genet. 1997, 6, 397–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamanti-Kandarakis, E.; Bartzis, M.; Bergiele, A.T.; Tsianateli, T.C.; Kouli, C.R. Microsatellite polymorphism (tttta)n at −528 base pairs of gene CYP11α influences hyperandrogenemia in patients with polycystic ovary syndrome. Fertil. Steril. 2000, 73, 735–741. [Google Scholar] [CrossRef]
- Pusalkar, M.; Meherji, P.; Gokral, J.; Chinnaraj, S.; Maitra, A. CYP11A1 and CYP17 promoter polymorphisms associate with hyperandrogenemia in polycystic ovary syndrome. Fertil. Steril. 2009, 92, 653–659. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Zhang, X.-L.; Xia, Y.-J.; Cao, Y.-X.; Wang, W.-J.; Xu, P.; Che, Y.-N.; Wu, X.-K.; Yi, L.; Gao, Q.; et al. Association between polymorphisms of the CYP11A1 gene and polycystic ovary syndrome in Chinese women. Mol. Biol. Rep. 2012, 39, 8379–8385. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, W.S.; Dabous, E.; Gerguis, A. Association between polymorphisms of the CYP11A1 gene and polycystic ovary syndrome in egyptian female. Res. J. Appl. Biotechnol. 2016, 2, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Huang, Y.; Lin, H. Estrogen disorders: Interpreting the abnormal regulation of aromatase in granulosa cells (Review). Int. J. Mol. Med. 2021, 47, 1–12. [Google Scholar] [CrossRef]
- Zaidi, S.K.; Shen, W.-J.; Cortez, Y.; Bittner, S.; Bittner, A.; Arshad, S.; Huang, T.-T.; Kraemer, F.B.; Azhar, S. SOD2 deficiency-induced oxidative stress attenuates steroidogenesis in mouse ovarian granulosa cells. Mol. Cell. Endocrinol. 2020, 519, 110888. [Google Scholar] [CrossRef]
- Reddy, K.R.; Deepika, M.L.N.; Supriya, K.; Latha, K.P.; Rao, S.S.L.; Rani, V.U.; Jahan, P. CYP11A1 microsatellite (tttta)n polymorphism in PCOS women from South India. J. Assist. Reprod. Genet. 2014, 31, 857–863. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.; Hasan, J.; Joseph, D.N. Association Between Polycystic Ovary Syndrome and Polymorphisms of CYP11A Gene Among Sample of Iraqi Women Introduction: Molecular Analysis: Gene selection Polymerase chain reaction (PCR). J. Univ. Shanghai Sci. Technol. 2020, 22, 712–725. [Google Scholar]
- Jiao, X.; Chen, W.; Zhang, J.; Wang, W.; Song, J.; Chen, D.; Zhu, W.; Shi, Y.; Yu, X. Variant Alleles of the ESR1, PPARG, HMGA2, and MTHFR genes are associated with polycystic ovary syndrome risk in a chinese population: A case-control study. Front. Endocrinol. 2018, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Asuncioón, M.; Calvo, R.M.; Millaán, J.L.S.; Sancho, J.; Avila, S.; Escobar-Morreale, H.F. A prospective study of the prevalence of the polycystic ovary syndrome in unselected caucasian women from Spain. Clin. Endocrinol. Metab. 2000, 85, 2434–2438. [Google Scholar] [CrossRef] [Green Version]
- Millán, J.S. Role of the pentanucleotide (tttta)n polymorphism in the promoter of the CYP11a gene in the pathogenesis of hirsutism. Fertil. Steril. 2001, 75, 797–802. [Google Scholar] [CrossRef]
- Li, T.; Guijin, Z. Role of the pentanucleotide (tttta)n polymorphisms ofCYP11α gene in the pathogenesis of hyperandrogenism in chinese women with polycystic ovary syndrome. J. Huazhong Univ. Sci. Technol. 2005, 25, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.F.; Bao, H.C.; Zhang, N.; Gu, H.E.; Chen, Z.J. Evaluation of association between the CYP11α promoter pen-tannucleotide (TTTTA)n polymorphism and polycystic ovarian syndrome among Han Chinese women. Neuroendocrinol. Lett. 2009, 30, 56–60. [Google Scholar]
- Pérez, M.S.; Cerrone, G.; Benencia, H.; Márquez, N.; De Piano, E.; Frechtel, G.D. Polymorphism in CYP11alpha and CYP17 genes and the etiology of hyperandrogenism in patients with polycystic ovary syndrome. Medicina 2008, 68, 129–134. [Google Scholar]
- Prazáková, S.; Vanková, M.; Bradnová, O.; Lukásová, P.; Vcelák, J.; Dvoráková, K.; Vondra, K.; Vrbíková, J.; Bendlová, B. (TTTTA), polymorphism in the promoter of the CYP11A1 gene in the pathogenesis of polycystic ovary syndrome. Cas. Lek. Ceskych 2010, 149, 520–525. [Google Scholar]
- Yu, M.; Feng, R.; Sun, X.; Wang, H.; Wang, H.; Sang, Q.; Jin, L.; He, L.; Wang, L. Polymorphisms of pentanucleotide repeats (tttta)n in the promoter of CYP11A1 and their relationships to polycystic ovary syndrome (PCOS) risk: A meta-analysis. Mol. Biol. Rep. 2014, 41, 4435–4445. [Google Scholar] [CrossRef]
- Shen, W.; Li, T.; Hu, Y.; Liu, H.; Song, M. Common polymorphisms in the CYP1A1 and CYP11A1 genes and polycystic ovary syndrome risk: A meta-analysis and meta-regression. Arch. Gynecol. Obstet. 2013, 289, 107–118. [Google Scholar] [CrossRef]
- Gaasenbeek, M.; Powell, B.L.; Sovio, U.; Haddad, L.; Gharani, N.; Bennett, A.; Groves, C.J.; Rush, K.; Goh, M.J.; Conway, G.S.; et al. Large-scale analysis of the relationship between CYP11A promoter variation, polycystic ovarian syndrome, and serum testosterone. J. Clin. Endocrinol. Metab. 2004, 89, 2408–2413. [Google Scholar] [CrossRef] [Green Version]
- Rosenfield, R.L.; Barnes, R.B.; Cara, J.F.; Lucky, A.W. Dysregulation of cytochrome P450c17α as the cause of polycystic ovarian syndrome. Supported in part by grants HD-06308 and Rr-00055 from the United States Public Health Service, Bethesda, Maryland. Fertil. Steril. 1990, 53, 785–791. [Google Scholar] [CrossRef]
- Beştaş, A.; Bolu, S.; Unal, E.; Karakaya, A.A.; Eröz, R.; Tekin, M.; Haspolat, Y.K. A rare cause of delayed puberty and primary amenorrhea: 17α-hydroxylase enzyme deficiency. Endocrine 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Echiburú, B.; Pérez-Bravo, F.; Maliqueo, M.; Sánchez, F.; Crisosto, N.; Sir-Petermann, T. Polymorphism T → C (−34 base pairs) of gene CYP17 promoter in women with polycystic ovary syndrome is associated with increased body weight and insulin resistance: A preliminary study. Metabolism 2008, 57, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Mutib, M.T.; Hamdan, F.B.; Al-Salihi, A.R. Effect of CYP19 Gene on Polycystic Ovary Syndrome Phenotype in Iraqi Women. iasj.net. Iraqi J. Med. Sci. 2015, 13, 272–278. [Google Scholar]
- Mohammad, M.; Al-Awadi, S.; Omran, M. Association Between Polycystic Ovary Syndrome and Genetic Polymorphisms of CYP 17 Gene in Iraqi Women. Iraqi J. Biothechnol. 2015, 14, 99–110. [Google Scholar]
- Kaur, R.; Kaur, T.; Kaur, A. Genetic association study from North India to analyze association of CYP19A1 and CYP17A1 with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2018, 35, 1123–1129. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bartzis, M.I.; Zapanti, E.D.; Spina, G.G.; Filandra, F.A.; Tsianateli, T.C.; Bergiele, A.T.; Kouli, C.R. Polymorphism T→C (−34 bp) of gene CYP17 promoter in Greek patients with polycystic ovary syndrome. Fertil. Steril. 1999, 71, 431–435. [Google Scholar] [CrossRef]
- Rahimi, Z.; Mohammadi, E. The CYP17 MSP AI (T-34C) and CYP19A1 (Trp39Arg) variants in polycystic ovary syndrome: A case-control study. Int. J. Reprod. Biomed. 2019, 17, 193–200. [Google Scholar] [CrossRef]
- Lone, N.M.; Babar, S.; Sultan, S.; Malik, S.; Nazeer, K.; Riaz, S. Association of the CYP17 and CYP19 gene polymorphisms in women with polycystic ovary syndrome from Punjab, Pakistan. Gynecol. Endocrinol. 2021, 37, 456–461. [Google Scholar] [CrossRef]
- Ashraf, S.; Rasool, S.U.A.; Nabi, M.; Ganie, M.A.; Jabeen, F.; Rashid, F.; Amin, S. CYP17 gene polymorphic sequence variation is associated with hyperandrogenism in Kashmiri women with polycystic ovarian syndrome. Gynecol. Endocrinol. 2021, 37, 230–234. [Google Scholar] [CrossRef]
- Wang, L.; Niu, Y.-M.; Wu, S.-S.; Zhang, C.; Zhou, L.; Zuo, H.-X.; Wang, P. A study on the association between polymorphisms in the cytochrome P450 family 17 subfamily a member 1 gene region and type 2 diabetes mellitus in han chinese. Front. Endocrinol. 2018, 9, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Techatraisak, K.; Chayachinda, C.; Wongwananuruk, T.; Dangrat, C.; Indhavivadhana, S.; Rattanachaiyanont, M.; Thongnoppakhun, W. No association between CYP17 -34T/C polymorphism and insulin resistance in Thai polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2015, 41, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Unsal, T.; Konac, E.; Yesilkaya, E.; Yilmaz, A.; Bideci, A.; Onen, H.I.; Cinaz, P.; Menevse, A. Genetic polymorphisms of FSHR, CYP17, CYP1A1, CAPN10, INSR, SERPINE1 genes in adolescent girls with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2009, 26, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahsarmiller, M.; Boots, L.; Bartolucci, A.; Azziz, R. Role of a CYP17 polymorphism in the regulation of circulating dehydroepiandrosterone sulfate levels in women with polycystic ovary syndrome. Fertil. Steril. 2004, 82, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.H.; Waterworth, D.; Patel, K.; White, D.; Little, J.; Novelli, P.; Franks, S.; Williamson, R. Polycystic ovaries and premature male pattern baldness are associated with one allele of the steroid metabolism gene CYP17. Hum. Mol. Genet. 1994, 3, 1873–1876. [Google Scholar] [CrossRef] [PubMed]
- Crocitto, L.E.; Feigelson, H.S.; Yu, M.C.; Kolonel, L.N.; Henderson, B.E.; Coetzee, G.A. A polymorphism in intron 6 of the CYP17 gene. Clin. Genet. 2008, 52, 68–69. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Iwao, K.; Ikeda, N.; Egawa, C.; Noguchi, S. Genetic polymorphism in CYP17 and breast cancer risk in Japanese women. Eur. J. Cancer 2000, 36, 2375–2379. [Google Scholar] [CrossRef]
- Baek, K.-H.; Park, J.-M.; Lee, E.-J.; Ramakrishna, S.; Cha, D.-H. Association study for single nucleotide polymorphisms in the CYP17A1 gene and polycystic ovary syndrome. Int. J. Mol. Med. 1998, 22, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Bardia, A.; Gucalp, A.; Dacosta, N.; Gabrail, N.; Danso, M.; Ali, H.; Blackwell, K.L.; Carey, L.A.; Eisner, J.R.; Baskin-Bey, E.S.; et al. Phase 1 study of seviteronel, a selective CYP17 lyase and androgen receptor inhibitor, in women with estrogen receptor-positive or triple-negative breast cancer. Breast Cancer Res. Treat. 2018, 171, 111–120. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, H.; Gao, M.; Tang, Z.; Guo, D.; Zhang, X.; Wang, Z.; Li, R.; Liu, Y.; Sun, W.; et al. Association between CYP17 T-34C rs743572 and breast cancer risk. Oncotarget 2017, 9, 4200–4213. [Google Scholar] [CrossRef] [Green Version]
- Ferzoco, R.M.; Ruddy, K.J. The Epidemiology of Male Breast Cancer. Curr. Oncol. Rep. 2015, 18, 1–6. [Google Scholar] [CrossRef]
- Effah, C.Y.; Wang, L.; Agboyibor, C.; Drokow, E.K.; Yu, S.; Wang, W.; Wu, Y. Polymorphism in the Androgen Biosynthesis Gene (CYP17), a Risk for Prostate Cancer: A Meta-Analysis. Am. J. Men’s Health 2020, 14, 1557988320959984. [Google Scholar] [CrossRef] [PubMed]
- Omear, H.A.; Al-assie, A.H. Plymorphism of CYP17 for Polycystic Ovarian Syndrome in Women of Salah Al-Din Provence/Iraq. J. Biotechnol. Res. Cent. 2014, 8, 50–54. [Google Scholar] [CrossRef]
- Louwers, Y.; Stolk, L.; Uitterlinden, A.; Laven, J. Cross-ethnic meta-analysis of genetic variants for polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E2006–E2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulun, S.E.; Yang, S.; Fang, Z.; Gurates, B.; Tamura, M.; Zhou, J.; Sebastian, S. Role of aromatase in endometrial disease. J. Steroid Biochem. Mol. Biol. 2001, 79, 19–25. [Google Scholar] [CrossRef]
- Xita, N.; Georgiou, I.; Lazaros, L.; Psofaki, V.; Kolios, G.; Tsatsoulis, A. The role of sex hormone-binding globulin and androgen receptor gene variants in the development of polycystic ovary syndrome. Hum. Reprod. 2008, 23, 693–698. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, L.H.; Prag, J. Human necrobacillosis, with emphasis on lemierre’s syndrome. Clin. Infect. Dis. 2000, 31, 524–532. [Google Scholar] [CrossRef]
- Altmäe, S.; Haller, K.; Peters, M.; Saare, M.; Hovatta, O.; Stavreus-Evers, A.; Velthut, A.; Karro, H.; Metspalu, A.; Salumets, A. Aromatase gene (CYP19A1) variants, female infertility and ovarian stimulation outcome: A preliminary report. Reprod. Biomed. Online 2009, 18, 651–657. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, W.-Y.; Choi, J.-Y.; Lee, S.S.; Shin, J.-G. Identification of CYP19A1 single-nucleotide polymorphisms and their haplotype distributions in a Korean population. J. Hum. Genet. 2010, 55, 189–193. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Doherty, J.A.; Shu, X.O.; Akbari, M.R.; Chen, C.; De Vivo, I.; DeMichele, A.; Garcia-Closas, M.; Goodman, M.T.; Haiman, C.A.; et al. Two estrogen-related variants in CYP19A1 and endometrial cancer risk: A pooled analysis in the Epidemiology of Endometrial Cancer Consortium. Cancer Epidemiol. Prev. Biomark. 2009, 18, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lu, X.; Wang, D.; Qu, W.; Li, W.; Xu, X.; Huang, Q.; Han, X.; Lv, J. CYP19 gene variant confers susceptibility to endometriosis-associated infertility in Chinese women. Exp. Mol. Med. 2014, 46, e103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazaros, L.A.; Hatzi, E.G.; Xita, N.V.; Makrydimas, G.V.; Kaponis, A.I.; Takenaka, A.; Kosmas, I.P.; Sofikitis, N.V.; Stefos, T.I.; Zikopoulos, K.A.; et al. Aromatase (CYP19) gene variants influence ovarian response to standard gonadotrophin stimulation. J. Assist. Reprod. Genet. 2012, 29, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, X.-L.; Zhang, C.-W.; Xu, P.; Liang, F.-J.; Che, Y.-N.; Xia, Y.-J.; Cao, Y.-X.; Wu, X.-K.; Wang, W.-J.; et al. SNP rs2470152 in CYP19 is correlated to aromatase activity in Chinese polycystic ovary syndrome patients. Mol. Med. Rep. 2011, 5, 245–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazaros, L.; Xita, N.; Hatzi, E.; Takenaka, A.; Kaponis, A.; Makrydimas, G.; Sofikitis, N.; Stefos, T.; Zikopoulos, K.; Georgiou, I. CYP19gene variants affect the assisted reproduction outcome of women with polycystic ovary syndrome. Gynecol. Endocrinol. 2013, 29, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, X.L.; Xie, G.B.; Zhang, C.W.; Shen, S.M.; Cao, Y.X.; Wang, W.J.; Che, Y.N.; Xia, Y.J.; Wu, X.K.; et al. The (TTTA) n polymorphism in intron 4 of CYP19 and the polycystic ovary syndrome risk in a Chinese population. Mol. Biol. Rep. 2013, 40, 5041–5047. [Google Scholar] [CrossRef]
- Dou, Q.; Tan, L.; Ma, L.-Y.; Sun, Y.-P. The relationship between the CYP19 alleles rs727479A/C, rs700518A/G, and rs700519C/T and pregnancy outcome after assisted reproductive technology in patients with polycystic ovary syndrome in a Chinese population: A population-based study. Kaohsiung J. Med Sci. 2017, 33, 558–566. [Google Scholar] [CrossRef]
- Mehdizadeh, A.; Kalantar, S.M.; Sheikhha, M.H.; Aali, B.S.; Ghanei, A. Association of SNP rs.2414096 CYP19 gene with polycystic ovarian syndrome in Iranian women. Int. J. Reprod. Biomed. 2017, 15, 491–496. [Google Scholar] [CrossRef] [Green Version]
- Feng-Jing, L.; Sun, J.; Ge, H.-J.; Cao, Y.-X.; Wu, X.-K.; Liang, F.-J.; Sun, H.-X.; Xiao-Ke, W.; Yi, L.; Wu, Z.-W.; et al. Association between CYP19 gene SNP rs2414096 Polymorphism and polycystic ovary syndrome in Chinese women. BMC Med. Genet. 2009, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Xita, N.; Lazaros, L.; Georgiou, I.; Tsatsoulis, A. CYP19 gene: A genetic modifier of polycystic ovary syndrome phenotype. Fertil. Steril. 2010, 94, 250–254. [Google Scholar] [CrossRef]
- Che, X.; Jian, F.; Chen, C.; Liu, C.; Liu, G.; Feng, W. PCOS serum-derived exosomal miR-27a-5p stimulates endometrial cancer cells migration and invasion. J. Mol. Endocrinol. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Lees, B.; Hampton, J.M.; Trentham-Dietz, A.; Newcomb, P.; Spencer, R. A population-based study of causes of death after endometrial cancer according to major risk factors. Gynecol. Oncol. 2021, 160, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Cui, J.; Goodarzi, M.O. Polycystic Ovary Syndrome and Risk of Type 2 Diabetes, Coronary Heart Disease, and Stroke. Diabetes 2020, 70, 627–637. [Google Scholar] [CrossRef]
- Ayyob, A.N.; Al-Badran, A.I.; Abood, R.A. Association of TTTA polymorphism in CYP19 gene with endometrial and ovarian cancers risk in Basrah. Gene Rep. 2019, 16, 100453. [Google Scholar] [CrossRef]
- Harris, H.R.; Terry, K.L. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: A systematic review. Fertil. Res. Pract. 2016, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kusum, K.; Patel, S.; Chaube, R.; Ashish, A.; Rai, S. Aromatase gene polymorphism (rs2470152) in Polycystic Ovary Syndrome patients of eastern Uttar Pradesh. J. Clin. Diagn. Res. 2020, 3–8. [Google Scholar] [CrossRef]
- Mostafa, R.A.; Al-Sherbeeny, M.M.; Fahmy, A.A.; Farghali, M.M.; Abdel-Fatah, M.A.; Mahran, M.Z. Relation between aromatase gene CYP19 variation and hyperandrogenism in Polycystic Ovary Syndrome Egyptian women. J. Infertil. Reprod. Biol. 2016, 4, 1–5. [Google Scholar]
- Araújo, B.S.; Baracat, M.C.P.; Simões, R.D.S.; Nuñes, C.D.O.; Maciel, G.A.R.; Lobo, R.A.; Soares-Jr, J.M.; Baracat, E.C. Kisspeptin Influence on Polycystic Ovary Syndrome—A Mini Review. Reprod. Sci. 2020, 27, 455–460. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef]

| Gene Name | SNP/STR Allele | Study Design | Country | Ethnicity | SS; Case/Control | Genotype Methods | Diagnostic Criteria | Clinical Characteristics | Risk Association | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| CYP11A1 | (TTTTA)n n = 4 *, 6, 8, 9 | Case–control study | Greece | Greek | 80/90 | PCR | NIH/NICHD | Hyperandrogenism | The pentanucleotides allele with 4 repeats (n = 4) is associated with higher serum total testosterone levels in PCOS. | [45] |
| CYP11A1 | (TTTTA)n n = 4, 6 *, 8, 9, 10, 12 | Case–control study | India | Asian | 100/100 | PCR | Rotterdam | Hyperandrogenism | A significant association of the pentanucleotides allele with 6 repeats (n = 6) with total testosterone levels is observed in PCOS group. | [46] |
| CYP11A1 | rs4077582 | Case–control study | Egypt | Egyptians | 53/53 | PCR–RFLP | Rotterdam | Diabetes mellitus, hirsutism, hyperandrogenism | The incidence of this polymorphism was positively associated with PCOS. | [48] |
| CYP11A1 | (TTTTA)n n = 2–16, 8 * | Case–control study | India | Asian | 267/275 | PCR-PAGE | Rotterdam | Hyperandrogenism | There was a significant increase in the pentanucleotides allele with 8 repeats (n = 8) with higher testosterone levels in PCOS. | [51] |
| CYP11A1 | (AAAAT)n n = 3, 5 *, 6, 7, 8 | Case–control study | Iraq | Asian | 74/58 | PCR/sequencing | Rotterdam | Polycystic ovaries oligovulation, hyperandrogenism | There was a significant relationship between the pentanucleotides allele with 5 repeats (n = 5) and PCOS. | [52] |
| CYP11A1 | (TTTTA)n n = 4, 6, 8, 9 | Case–control study | Spain | Caucasian | 92/33 | PCR/ sequencing | NIH/NICHD | Hyperandrogenism Hirsutism | No association was found between (TTTTA)n in CYP11A1 and hyperandrogenism. | [55] |
| CYP11A1 | (TTTTA)n n = 4, 6, 8, 9 | Case–control study | China | Asian | 96/78 | PCR | Rotterdam | Hyperandrogenism | No association was found between (TTTTA)n in CYP11A1 and hyperandrogenism. | [56] |
| CYP11A1 | (TTTTA)n n = 4, 8 | Case–control study | China | Asian | 125/121 | PCR | Rotterdam | Anovulation & Hyperandrogenism | There is no statistically significant difference. | [57] |
| CYP11A1 | (TTTTA)n n = 4, 6, 8 | Case–control study | Argentine | Non-Caucasian | 65/58 | PCR | NIH/NICHD | Hyperandrogenism | No statistically significant relationship was found between the pathogenesis of CYP11A1 and PCOS. | [58] |
| CYP11A1 | (TTTTA)n n = 4 | Case–control study | Czech | Caucasian | 256/109 | PCR | Rotterdam | Hyperandrogenism | There is no statistically significant difference. | [59] |
| Gene Name | SNP/STR Allele | Study Design | Country | Ethnicity | SS; Case/Control | Genotype-Methods | Diagnostic Criteria | Clinical Characteristics | Risk Association | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| CYP17A1 | rs743572 | Case–control study | Chile | Caucasian | 66/93 | PCR -RFLP | NIH/NICHD | hormonal and clinical evidence of PCOS | CYP17A1 is associated with metabolic pathway and obesity in women with PCOS | [65] |
| CYP17A1 | rs743572 | Case–control study | India | Asian | 100/100 | PCR-sequencing | Rotterdam | Anovulation | CYP17A1 was associated with hyperandrogenaemia | [46] |
| CYP17A1 | rs743572 | Case–control study | Iraq | Asian | 84/65 | PCR | - | Anovulation | Positive correlation | [85] |
| CYP17A1 | rs743572 | Case–control study | Iraq | Asian | 61/30 | PCR-RFLP | Rotterdam | PCOS criteria | There is a correlation between the –34 T > C allele and increasing HDL | [67] |
| CYP17A1 | rs743572 | Case–control study | India | Asian | 250/250 | PCR-RFLP | Rotterdam | Anovulation | CYP17A1 34 T > C occurrence had a significant relation with PCOS patients | [68] |
| CYP17A1 | rs743572 | Case–control study | Iran | Asian | 50/109 | PCR-RFLP | Rotterdam | PCOS criteria | Polymorphism in CYP17A1 is associated with the risk of PCOS | [70] |
| CYP17A1 | rs743572 | Case–control study | Pakistan | Asian | 204/100 | PCR-RFLP | Rotterdam | PCOS criteria | rs743572 is correlated with the occurrence of PCOS | [71] |
| CYP17A1 | rs743572 | Case–control study | India | Asian | 394/306 | PCR-RFLP | Rotterdam | Hyperandrogenism | Confirmation of the role of SNP in hyperandrogenism with increasing androgen | [72] |
| CYP17A1 | rs1004467, rs17115149, rs12413409 | Case–control study | China | Asian | 440/1320 | PCR-Genotype | - | Type two diabetes Mellitus | CYP17A1 might be considered as a risk factor to T2DM | [73] |
| CYP17A1 | rs743572 | Cross-sectional study | Thailand | Asian | 210 | PCR-RFLP | Rotterdam | PCOS criteria | There was no significant association between –34 T > C and insulin resistance | [74] |
| CYP17A1 | rs743572 | Case–control study | Turkey | Asian | 44/50 | PCR-RFLP | Rotterdam | PCOS criteria | There was no association among case and control groups in allele –34 T > C | [75] |
| CYP17A1 | rs743572 | Case–control study | America | Caucasian | 259/161 | PCR | NIH/NICHD | PCOS criteria | There was no association with the development of PCOS and CYP17A1 C/T | [76] |
| Gene Name | SNP/STR Allele | Study Design | Country | Ethnicity | SS; Case/Control | Genotype Methods | Diagnostic Criteria | Clinical Characteristics | Risk Association | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| CYP19A1 | (TTTA)n n = 7 *–12 | Case–control study | Greece | Greek | 300/300 | PCR-PAGE | - | Male factor-infertility | There was a significant association between the tetranucleotide allele with 7 repeats (n = 7) and FSH level. | [94] |
| CYP19A1 | rs2470152 | Case–control study | China | Asian | 364/297 | PCR- RFLP | Rotterdam | PCOS features | rs2470152 polymorphism was associated with aromatase activity | [95] |
| CYP19A1 | (TTTA)n n = 7 *–12 | Case–control study | Greece | Greek | 132/200 | PCR-PAGE | NIH/NICHD | Ovulatory-dysfunction | Carriers with the tetranucleotide allele with 7 repeats (n = 7) presented higher testosterone levels compared to non-carriers with the tetranucleotide allele with 7 repeats (n = 7) | [96] |
| CYP19A1 | (TTTA)n n = 7, 8, 10, 11, 12, 13 | Case–control study | China | Asian | 222/281 | PCR-Capillary electrophoresis | Rotterdam | PCOS features | PCOS patients showed a higher frequency of short alleles compared to controls. | [97] |
| CYP19A1 | rs727479 rs700518 rs700519 | Case–control study | China | Asian | 150/143 | PCR-DHPLC | Rotterdam | PCOS features | The CGT and CAT haplotypes were desirable to fertility result in PCOS patients | [98] |
| CYP19A1 | rs2414096 | Case–control study | Iran | Asian | 70/70 | PCR-RFLP | Rotterdam | PCOS features | There was a positive relation between rs2414096 alleles in PCOS group | [99] |
| CYP19A1 | rs700519 rs2414096 | Case–control study | India | Asian | 250/250 | PCR-RFLP | Rotterdam | PCOS features | No significant association was found between alleles and PCOS | [68] |
| CYP19A1 | rs2470152 | Case–control study | India | Asian | 120/180 | PCR-RFLP | Rotterdam | PCOS feature | Allele rs2470152 was significantly higher in women with PCOS | [107] |
| CYP19A1 | rs2236722 | Case–control study | Iran | Asian | 50/109 | PCR | Rotterdam | Ovarian dysfunction | Incidence of rs2236722 polymorphism may not be associated with an increased risk of PCOS | [70] |
| CYP19A1 | rs2414096 | Case–control study | Egypt | Egyptians | 30/30 | PCR-RFLP | Rotterdam | PCOS criteria | Allele rs2414096 was associated with aromatase deficiency and hyperandrogenism | [109] |
| CYP19A1 | rs2414096 | Case–control study | Iraq | Asian | 84/65 | PCR-RFLP | Rotterdam | Oligomenorrhea | Allele rs2414096 present was related to hyperandrogenism in case group | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidarzadehpilehrood, R.; Pirhoushiaran, M.; Abdollahzadeh, R.; Binti Osman, M.; Sakinah, M.; Nordin, N.; Abdul Hamid, H. A Review on CYP11A1, CYP17A1, and CYP19A1 Polymorphism Studies: Candidate Susceptibility Genes for Polycystic Ovary Syndrome (PCOS) and Infertility. Genes 2022, 13, 302. https://doi.org/10.3390/genes13020302
Heidarzadehpilehrood R, Pirhoushiaran M, Abdollahzadeh R, Binti Osman M, Sakinah M, Nordin N, Abdul Hamid H. A Review on CYP11A1, CYP17A1, and CYP19A1 Polymorphism Studies: Candidate Susceptibility Genes for Polycystic Ovary Syndrome (PCOS) and Infertility. Genes. 2022; 13(2):302. https://doi.org/10.3390/genes13020302
Chicago/Turabian StyleHeidarzadehpilehrood, Roozbeh, Maryam Pirhoushiaran, Rasoul Abdollahzadeh, Malina Binti Osman, Maryam Sakinah, Norshariza Nordin, and Habibah Abdul Hamid. 2022. "A Review on CYP11A1, CYP17A1, and CYP19A1 Polymorphism Studies: Candidate Susceptibility Genes for Polycystic Ovary Syndrome (PCOS) and Infertility" Genes 13, no. 2: 302. https://doi.org/10.3390/genes13020302