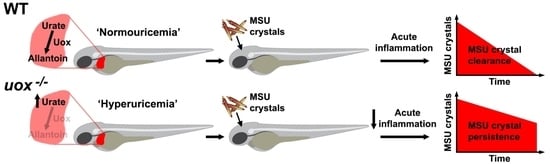
Uricase-Deficient Larval Zebrafish with Elevated Urate Levels Demonstrate Suppressed Acute Inflammatory Response to Monosodium Urate Crystals and Prolonged Crystal Persistence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry and Fish Lines
2.2. Phylogenetic Analysis and Multiple Sequence Alignment (MSA)
2.3. Generation of Uox Mutants and Genotyping
2.4. Urate Quantification
2.5. MSU Crystal Synthesis and Microinjection into Zebrafish Larvae
2.6. RNA Extraction and Quantitative PCR (qPCR)
2.7. Whole-Mount In Situ Hybridization (WMISH)
2.8. Flow Cytometry
2.9. Immunostaining for Neutrophil Recruitment
2.10. Live Imaging
2.11. MSU Crystal Quantification Using Confocal Reflection Microscopy
2.12. Data and Statistical Analysis
3. Results
3.1. Zebrafish Uricase Is Highly Conserved
3.2. Generating a Zebrafish Uox Knockout Line
3.3. uox−/− Larvae Possess Elevated Levels of Urate While Adults Are Viable, Healthy and Fertile
3.4. MSU Crystals Persist When Microinjected into uox−/− Larvae and Can Be Quantified Using Reflection Confocal Microscopy
3.5. uox−/− Larvae Maintain Normal Leucocyte Abundance but Show Dampened Neutrophil Recruitment to MSU Crystal Challenge
3.6. Persisting MSU Crystal Aggregates in uox−/− Larvae form Structures That Are Dominated by Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Y.; Wu, Q.; Wang, H.; Zhang, S.; Jiang, Y.; Gong, T.; Xu, X.; Chang, Q.; Niu, K.; Zhao, Y. Global, regional and national burden of gout, 1990–2017: A systematic analysis of the Global Burden of Disease Study. Rheumatology 2020, 59, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J.; Harper, J.L. Innate inflammation and resolution in acute gout. Immunol. Cell Biol. 2010, 88, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Chhana, A.; Dalbeth, N. The gouty tophus: A review. Curr. Rheumatol. Rep. 2015, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Aati, O.; Kalluru, R.; Gamble, G.D.; Horne, A.; Doyle, A.J.; McQueen, F.M. Relationship between structural joint damage and urate deposition in gout: A plain radiography and dual-energy CT study. Ann. Rheum. Dis. 2015, 74, 1030–1036. [Google Scholar] [CrossRef]
- Dalbeth, N.; Clark, B.; Gregory, K.; Gamble, G.; Sheehan, T.; Doyle, A.; McQueen, F.M. Mechanisms of bone erosion in gout: A quantitative analysis using plain radiography and computed tomography. Ann. Rheum. Dis. 2009, 68, 1290–1295. [Google Scholar] [CrossRef]
- Eason, A.; House, M.E.; Vincent, Z.; Knight, J.; Tan, P.; Horne, A.; Gamble, G.D.; Doyle, A.J.; Taylor, W.J.; Dalbeth, N. Factors associated with change in radiographic damage scores in gout: A prospective observational study. Ann. Rheum. Dis. 2016, 75, 2075–2079. [Google Scholar] [CrossRef]
- Sapsford, M.; Gamble, G.D.; Aati, O.; Knight, J.; Horne, A.; Doyle, A.J.; Dalbeth, N. Relationship of bone erosion with the urate and soft tissue components of the tophus in gout: A dual energy computed tomography study. Rheumatology 2017, 56, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Perez-Ruiz, F.; Martínez-Indart, L.; Carmona, L.; Herrero-Beites, A.M.; Pijoan, J.I.; Krishnan, E. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann. Rheum. Dis. 2014, 73, 177–182. [Google Scholar] [CrossRef]
- Vincent, Z.L.; Gamble, G.; House, M.; Knight, J.; Horne, A.; Taylor, W.J.; Dalbeth, N. Predictors of Mortality in People with Recent-onset Gout: A Prospective Observational Study. J. Rheumatol. 2017, 44, 368–373. [Google Scholar] [CrossRef]
- Dalbeth, N.; Pool, B.; Gamble, G.D.; Smith, T.; Callon, K.E.; McQueen, F.M.; Cornish, J. Cellular characterization of the gouty tophus: A quantitative analysis. Arthritis Rheum. 2010, 62, 1549–1556. [Google Scholar] [CrossRef]
- Harris, M.D.; Siegel, L.B.; Alloway, J.A. Gout and hyperuricemia. Am. Fam. Physician 1999, 59, 925–934. [Google Scholar] [PubMed]
- Williams, L.A. The History, Symptoms, Causes, Risk Factors, Types, Diagnosis, Treatments, and Prevention of Gout, Part 2. Int. J. Pharm. Compd. 2019, 23, 14–21. [Google Scholar] [PubMed]
- Iwata, H.; Nishio, S.; Yokoyama, M.; Matsumoto, A.; Takeuchi, M. Solubility of uric acid and supersaturation of monosodium urate: Why is uric acid so highly soluble in urine? J. Urol. 1989, 142, 1095–1098. [Google Scholar] [CrossRef]
- Ramazzina, I.; Folli, C.; Secchi, A.; Berni, R.; Percudani, R. Completing the uric acid degradation pathway through phylogenetic comparison of whole genomes. Nat. Chem. Biol. 2006, 2, 144–148. [Google Scholar] [CrossRef]
- Wu, X.W.; Muzny, D.M.; Lee, C.C.; Caskey, C.T. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J. Mol. Evol. 1992, 34, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Mangoff, S.C.; Milner, J.A. Oxonate-induced hyperuricemia and orotic aciduria in mice. Proc. Soc. Exp. Biol. Med. 1978, 157, 110–115. [Google Scholar] [CrossRef]
- Stavric, B.; Nera, E.A. Use of the uricase-inhibited rat as an animal model in toxicology. Clin. Toxicol. 1978, 13, 47–74. [Google Scholar] [CrossRef]
- Wu, X.; Wakamiya, M.; Vaishnav, S.; Geske, R.; Montgomery, C., Jr.; Jones, P.; Bradley, A.; Caskey, C.T. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc. Natl. Acad. Sci. USA 1994, 91, 742–746. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Hou, X.; Yuan, X.; Cui, L.; Liu, Z.; Li, X.; Ma, L.; Cheng, X.; Xin, Y.; Wang, C.; et al. Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int. 2018, 93, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Cook, S.A.; Akeson, E.C.; Calvano, C.; Johnson, K.R.; Mandell, J.; Hawes, N.L.; Bronson, R.T.; Roderick, T.H.; Davisson, M.T. Mouse paracentric inversion In(3)55Rk mutates the urate oxidase gene. Cytogenet. Cell Genet. 2001, 93, 77–82. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, N.; Dong, X.; Fan, N.; Wang, L.; Xu, Y.; Chen, H.; Duan, W. Uricase-deficient rat is generated with CRISPR/Cas9 technique. PeerJ 2020, 8, e8971. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yu, Y.; Qin, W.; Fan, N.; Qi, Y.; Chen, H.; Duan, W. Uricase-deficient rats with similarly stable serum uric acid to human’s are sensitive model animals for studying hyperuricemia. PLoS ONE 2022, 17, e0264696. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.J.; Sanderson, L.E.; Crosier, K.E.; Crosier, P.S. Mitochondrial metabolism, reactive oxygen species, and macrophage function-fishing for insights. J. Mol. Med. 2014, 92, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Flores, M.V.; Storm, T.; Crosier, K.; Crosier, P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev. Biol. 2007, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Renshaw, S.A.; Loynes, C.A.; Trushell, D.M.; Elworthy, S.; Ingham, P.W.; Whyte, M.K. A transgenic zebrafish model of neutrophilic inflammation. Blood 2006, 108, 3976–39785. [Google Scholar] [CrossRef]
- Ellett, F.; Pase, L.; Hayman, J.W.; Andrianopoulos, A.; Lieschke, G.J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011, 117, e49–e56. [Google Scholar] [CrossRef] [Green Version]
- Linnerz, T.; Hall, C.J. The Diverse Roles of Phagocytes during Bacterial and Fungal Infections and Sterile Inflammation: Lessons From Zebrafish. Front. Immunol. 2020, 11, 1094. [Google Scholar] [CrossRef]
- Hall, C.J.; Sanderson, L.E.; Lawrence, L.M.; Pool, B.; van der Kroef, M.; Ashimbayeva, E.; Britto, D.; Harper, J.L.; Lieschke, G.J.; Astin, J.W.; et al. Blocking fatty acid-fueled mROS production within macrophages alleviates acute gouty inflammation. J. Clin. Investig. 2018, 128, 1752–1771. [Google Scholar] [CrossRef] [Green Version]
- Britto, D.D.; Wyroba, B.; Chen, W.; Lockwood, R.A.; Tran, K.B.; Shepherd, P.R.; Hall, C.J.; Crosier, K.E.; Crosier, P.S.; Astin, J.W. Macrophages enhance Vegfa-driven angiogenesis in an embryonic zebrafish tumour xenograft model. Dis. Model Mech. 2018, 11, dmm035998. [Google Scholar] [CrossRef] [Green Version]
- Walton, E.M.; Cronan, M.R.; Beerman, R.W.; Tobin, D.M. The Macrophage-Specific Promoter mfap4 Allows Live, Long-Term Analysis of Macrophage Behavior during Mycobacterial Infection in Zebrafish. PLoS ONE 2015, 10, e0138949. [Google Scholar] [CrossRef]
- Kwan, K.M.; Fujimoto, E.; Grabher, C.; Mangum, B.D.; Hardy, M.E.; Campbell, D.S.; Parant, J.M.; Yost, H.J.; Kanki, J.P.; Chien, C.B. The Tol2kit: A multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 2007, 236, 3088–3099. [Google Scholar] [CrossRef]
- Meeker, N.D.; Hutchinson, S.A.; Ho, L.; Trede, N.S. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. Biotechniques 2007, 43, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.C.; Cheong, W.C.; Lim, L.S.; Li, M.H. A simple, high sensitivity mutation screening using Ampligase mediated T7 endonuclease I and Surveyor nuclease with microfluidic capillary electrophoresis. Electrophoresis 2012, 33, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Smith, T.; Nicolson, B.; Clark, B.; Callon, K.; Naot, D.; Haskard, D.O.; McQueen, F.M.; Reid, I.R.; Cornish, J. Enhanced osteoclastogenesis in patients with tophaceous gout: Urate crystals promote osteoclast development through interactions with stromal cells. Arthritis Rheum. 2008, 58, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Jowett, T.; Lettice, L. Whole-mount in situ hybridizations on zebrafish embryos using a mixture of digoxigenin- and fluorescein-labelled probes. Trends Genet. 1994, 10, 73–74. [Google Scholar] [CrossRef]
- Hall, C.; Flores, M.V.; Chien, A.; Davidson, A.; Crosier, K.; Crosier, P. Transgenic zebrafish reporter lines reveal conserved Toll-like receptor signaling potential in embryonic myeloid leukocytes and adult immune cell lineages. J. Leukoc. Biol. 2009, 85, 751–765. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.J.; Boyle, R.H.; Astin, J.W.; Flores, M.V.; Oehlers, S.H.; Sanderson, L.E.; Ellett, F.; Lieschke, G.J.; Crosier, K.E.; Crosier, P.S. Immunoresponsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating β-oxidation-dependent mitochondrial ROS production. Cell Metab. 2013, 18, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Li, H.; Chi, Z.; He, J. Loss of the RNA-binding protein Rbm15 disrupts liver maturation in zebrafish. J. Biol. Chem. 2020, 295, 11466–11472. [Google Scholar] [CrossRef]
- Linnerz, T.; Kanamala, M.; Astin, J.W.; Dalbeth, N.; Wu, Z.; Hall, C.J. Targeting Drugs to Larval Zebrafish Macrophages by Injecting Drug-Loaded Liposomes. J. Vis. Exp. 2020, 156, e60198. [Google Scholar] [CrossRef]
- Wu, Z.; Koh, B.; Lawrence, L.M.; Kanamala, M.; Pool, B.; Svirskis, D.; Dalbeth, N.; Astin, J.W.; Crosier, K.E.; Crosier, P.S.; et al. Liposome-Mediated Drug Delivery in Larval Zebrafish to Manipulate Macrophage Function. Zebrafish 2019, 16, 171–181. [Google Scholar] [CrossRef]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef]
- Lu, J.; Dalbeth, N.; Yin, H.; Li, C.; Merriman, T.R.; Wei, W.H. Mouse models for human hyperuricaemia: A critical review. Nat. Rev. Rheumatol. 2019, 15, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Joosten, L.A.B.; Crisan, T.O.; Bjornstad, P.; Johnson, R.J. Asymptomatic hyperuricaemia: A silent activator of the innate immune system. Nat. Rev. Rheumatol. 2020, 16, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Immler, R.; Pruenster, M.; Sellmayr, M.; Li, C.; von Brunn, A.; von Brunn, B.; Ehmann, R.; Wolfel, R.; Napoli, M.; et al. Soluble uric acid inhibits beta2 integrin-mediated neutrophil recruitment in innate immunity. Blood 2022, 139, 3402–3417. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Honarpisheh, M.; Li, C.; Sellmayr, M.; Lindenmeyer, M.; Bohland, C.; Romagnani, P.; Anders, H.J.; Steiger, S. Soluble Uric Acid Is an Intrinsic Negative Regulator of Monocyte Activation in Monosodium Urate Crystal-Induced Tissue Inflammation. J. Immunol. 2020, 205, 789–800. [Google Scholar] [CrossRef]
- Dalbeth, N.; House, M.E.; Aati, O.; Tan, P.; Franklin, C.; Horne, A.; Gamble, G.D.; Stamp, L.K.; Doyle, A.J.; McQueen, F.M. Urate crystal deposition in asymptomatic hyperuricaemia and symptomatic gout: A dual energy CT study. Ann. Rheum. Dis. 2015, 74, 908–911. [Google Scholar] [CrossRef]
- Martin, W.J.; Walton, M.; Harper, J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum. 2009, 60, 281–289. [Google Scholar] [CrossRef]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhofer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef]
- Schorn, C.; Janko, C.; Krenn, V.; Zhao, Y.; Munoz, L.E.; Schett, G.; Herrmann, M. Bonding the foe—NETting neutrophils immobilize the pro-inflammatory monosodium urate crystals. Front. Immunol. 2012, 3, 376. [Google Scholar] [CrossRef]
- Isles, H.M.; Loynes, C.A.; Alasmari, S.; Kon, F.C.; Henry, K.M.; Kadochnikova, A.; Hales, J.; Muir, C.F.; Keightley, M.C.; Kadirkamanathan, V.; et al. Pioneer neutrophils release chromatin within in vivo swarms. Elife 2021, 10, e68755. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linnerz, T.; Sung, Y.J.; Rolland, L.; Astin, J.W.; Dalbeth, N.; Hall, C.J. Uricase-Deficient Larval Zebrafish with Elevated Urate Levels Demonstrate Suppressed Acute Inflammatory Response to Monosodium Urate Crystals and Prolonged Crystal Persistence. Genes 2022, 13, 2179. https://doi.org/10.3390/genes13122179
Linnerz T, Sung YJ, Rolland L, Astin JW, Dalbeth N, Hall CJ. Uricase-Deficient Larval Zebrafish with Elevated Urate Levels Demonstrate Suppressed Acute Inflammatory Response to Monosodium Urate Crystals and Prolonged Crystal Persistence. Genes. 2022; 13(12):2179. https://doi.org/10.3390/genes13122179
Chicago/Turabian StyleLinnerz, Tanja, Yih Jian Sung, Leah Rolland, Jonathan W. Astin, Nicola Dalbeth, and Christopher J. Hall. 2022. "Uricase-Deficient Larval Zebrafish with Elevated Urate Levels Demonstrate Suppressed Acute Inflammatory Response to Monosodium Urate Crystals and Prolonged Crystal Persistence" Genes 13, no. 12: 2179. https://doi.org/10.3390/genes13122179