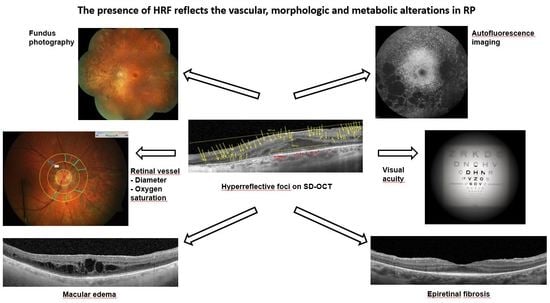
The Presence of Hyperreflective Foci Reflects Vascular, Morphologic and Metabolic Alterations in Retinitis Pigmentosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Retinal Vessel Imaging
2.3. Optical Coherence Tomography (OCT)
2.4. OCT Measurements
2.5. Statistical Analysis
3. Results
3.1. HRF in RP Patients against Controls (OCT Imaging)
3.2. Retinal Vessel Diameters in RP Patients against Controls
3.3. Retinal Vessel Oxygen Saturation Values in RP Patients against Controls
3.4. Further Structural Alterations Measured by OCT Imaging
IS-OS Line/Ellipsoid Zone (EZ)
3.5. Interactions between the Number of HRF and the Functional (BCVA), Structural (Retinal Vessel Diameters and EZ Length) and Metabolic Alterations (Retinal Vessel Oxygen Saturation Values) in RP Compared to Controls
4. Discussion
4.1. HRF in RP Patients
4.2. Attenuated Retinal Vessels in RP Patients
4.3. Metabolic Alterations in RP Patients
5. Conclusions
Limitations
- Relatively small number of cases due to the rarity of the disease were included in the study.
- One further limitation is the absence of follow up, which could have helped to reevaluate the progression taking into account the number of hyperreflective foci and visual acuity.
- Compared to the study of Chu-Hsuan Huang [39] we divided the groups into hyperreflective foci within the entire retina (HRF-ER) and the outer nuclear layer (HRF-ONL) without considering the foci in the choroid and their thickness. The study may also be limited due to the results of Hanumunthadu et al. [52] describing a decrease in the choroidal vascular index in RP patients when HRF were represented.
- Evaluation of the presence of HRF has been proposed in several studies to be done using the EDTRS grid of the macula. However, we decided to keep the data analysis using horizontal scans, so that to be able to take the presence of ME und ERF in RP into account.
- One remaining open question in the present study is whether the HRF is also present in healthy controls. The presence of HRF in normal eyes and with aging has not been discussed until now. Some possible explanations could be the decreased vessel dispensability, the ongoing vascular structural remodeling and also changes in viscoelastic properties occurring with ageing [83]. All of these are probably leading to metabolic changes throughout their lifetime [84].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liew, G.; Michaelides, M.; Bunce, C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 2014, 4, e004015. [Google Scholar] [CrossRef] [PubMed]
- Finger, R.P.; Fimmers, R.; Holz, F.G.; Scholl, H.P. Prevalence and causes of registered blindness in the largest federal state of Germany. Br. J. Ophthalmol. 2011, 95, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Keeffe, J.E.; McCarty, C.; Hassell, J.B.; Gilbert, A.G. Description and measurement of handicap caused by vision impairment. Aust. N.Z.J. Ophthalmol. 1999, 27, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Ammann, F.; Klein, D.; Franceschetti, A. Genetic and epidemiological investigations on pigmentary degeneration of the retina and allied disorders in Switzerland. J. Neurol. Sci. 1965, 2, 183–196. [Google Scholar] [CrossRef]
- Puech, B.; Kostrubiec, B.; Hache, J.C.; François, P. Epidemiology and prevalence of hereditary retinal dystrophies in the Northern France. J. Fr. Ophtalmol. 1991, 14, 153–164. [Google Scholar]
- Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 11, 40. [Google Scholar] [CrossRef]
- Hamel, C. Cone rod dystrophies. Orphanet J. Rare Dis. 2007, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Michaelides, M.; Hunt, D.; Moore, A.T. The genetics of inherited macular dystrophies. J. Med. Genet. 2003, 40, 641–650. [Google Scholar] [CrossRef]
- Hajali, M.; Fishman, G.; Anderson, R.J. The prevalence of cystoid macular oedema in retinitis pigmentosa patients determined by optical coherence tomography. Br. J. Ophthalmol. 2008, 92, 1065–1068. [Google Scholar] [CrossRef]
- Testa, F.; Rossi, S.; Colucci, R.; Gallo, B.; di Iorio, V.; della Corte, M.; Azzolini, C.; Melillo, P.; Simonelli, F. Macular abnormalities in Italian patients with retinitis pigmentosa. Br. J. Ophthalmol. 2014, 98, 946–950. [Google Scholar] [CrossRef] [Green Version]
- Adackapara, C.A.; Sunness, J.; Dibernardo, C.W.; Melia, B.M.; Dagnelie, G. Prevalence of cystoid macular edema and stability in oct retinal thickness in eyes with retinitis pigmentosa during a 48-week lutein trial. Retina 2008, 28, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, H.; Karacorlu, M.; Karacorlu, S. Intravitreal triamcinolone acetonide for treatment of cystoid macular oedema in patients with retinitis pigmentosa. Acta Ophthalmol. Scand. 2005, 83, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.A.; Brockhurs, R.; Gaudio, A.R.; Berson, E.L. The association between visual acuity and central retinal thickness in retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3349–3354. [Google Scholar] [CrossRef] [Green Version]
- Mitamura, Y.; Mitamura-Aizawa, S.; Nagasawa, T.; Katome, T.; Eguchi, H.; Naito, T. Diagnostic imaging in patients with retinitis pigmentosa. J. Med. Investig. 2012, 59, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iriyama, A.; Yanagi, Y. Fundus autofluorescence and retinal structure as determined by spectral domain optical coherence tomography, and retinal function in retinitis pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 333–339. [Google Scholar] [CrossRef]
- Aizawa, S.; Mitamura, Y.; Baba, T.; Hagiwara, A.; Ogata, K.; Yamamoto, S. Correlation between visual function and photoreceptor inner/outer segment junction in patients with retinitis pigmentosa. Eye 2009, 23, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Fishman, G.A. Electrophysiology and inherited retinal disorders. Doc. Ophthalmol. 1985, 60, 107–119. [Google Scholar] [CrossRef]
- Gouras, P.; Carr, R. Electrophysiological studies in early retinitis pigmentosa. Arch. Ophthalmol. 1964, 72, 104–110. [Google Scholar] [CrossRef]
- Birch, D.G.; Sandberg, M.A. Dependence of cone b-wave implicit time on rod amplitude in retinitis pigmentosa. Vis. Res. 1987, 27, 1105–1112. [Google Scholar] [CrossRef]
- Sandberg, M.A.; Weigel, D.C.; Rosner, B.; Berson, E.L. The relationship between visual field size and electroretinogram amplitude in retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1693–1698. [Google Scholar]
- Friesacher, A.; Lopez Torres, L.T.; Valmaggia, C.; Rüesch, R.; Todorova, M.G. Linking the Presence of Macular Oedema to Structural and Functional Alterations in Retinitis Pigmentosa. Klin. Monbl. Augenheilkd. 2021, 238, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Lopez Torres, L.T.; Türksever, C.; Schötzau, A.; Orgül, S.; Todorova, M.G. Peripapillary retinal vessel diameter correlates with mfERG alterations in retinitis pigmentosa. Acta Ophthalmol. 2015, 93, e527–e533. [Google Scholar] [CrossRef] [PubMed]
- Bojinova, R.I.; Türksever, C.; Schötzau, A.; Valmaggia, C.; Schorderet, D.F.; Todorova, M.G. Reduced metabolic function and structural alterations in inherited retinal dystrophies: Investigating the effect of peripapillary vessel oxygen saturation and vascular diameter on the retinal nerve fibre layer thickness. Acta Ophthalmol. 2017, 95, 252–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Volpe Waizel, M.; Scoll, H.; Todorova, M.G. Microvascular and metabolic alterations in retinitis pigmentosa and Stargardt disease. Acta Ophthalmol. 2021, 99, e1396–e1404. [Google Scholar] [CrossRef]
- Stefánsson, E.; Olafsdottir, O.B.; Eliasdottir, T.S.; Vehmeijer, W.; Einarsdottir, A.B.; Bek, T.; Torp, T.L.; Grauslund, J.; Eysteinsson, T.; Karlsson, R.A.; et al. Retinal oximetry: Metabolic imaging for diseases of the retina and brain. Prog. Retin. Eye Res. 2019, 70, 1–22. [Google Scholar] [CrossRef]
- Schweitzer, D.; Thamm, E.; Hammer, M.; Kraft, J. A New Method for the Measurement of Oxygen Saturation at the Human Ocular Fundus. Int. Ophthalmol. 2001, 23, 347–353. [Google Scholar] [CrossRef]
- Hammer, M.; Thamm, E.; Schweitzer, D. A simple algorithm for in vivo ocular fundus oximetry compensating for non-haemoglobin absorption and scattering. Phys. Med. Biol. 2002, 47, N233–N238. [Google Scholar] [CrossRef] [Green Version]
- Hardarson, S. Retinal oximetry. Acta Ophthalmol. 2013, 91, 1–47. [Google Scholar] [CrossRef]
- Geirsdottir, A.; Palsson, O.; Hardarson, S.H.; Olafsdottir, O.B.; Kristjansdottir, J.V.; Stefánsson, E. Retinal vessel oxygen saturation in healthy individuals. Investig. Ophthalmol. Vis. Sci. 2012, 13, 5433–5442. [Google Scholar] [CrossRef] [Green Version]
- Türksever, C.; Orgül, S.; Todorova, M.G. Reproducibility of retinal oximetry measurements in healthy and diseased retinas. Acta Ophthalmol. 2015, 93, e439–e445. [Google Scholar] [CrossRef]
- Türksever, C.; Valmaggia, C.; Orguel, S.; Schorderet, D.F.; Todorova, M.G. Retinal vessel oxygen saturation in retinitis pigmentosa patients. Acta Ophthalmol. 2013, 91, eTO23. [Google Scholar] [CrossRef]
- Todorova, M.G.; Türksever, C.; Schorderet, D.F.; Valmaggia, C. Retinal vessel Oxygen Saturation in patients suffering from inherited diseases of the retina. Klin. Monbl. Augenheilkd. 2014, 231, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Türksever, C.; Valmaggia, C.; Orgül, S.; Schorderet, D.F.; Flammer, J.; Todorova, M.G. Retinal vessel oxygen saturation and its correlation with structural changes in retinitis pigmentosa. Acta Ophthalmol. 2014, 92, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Todorova, M.G.; Türksever, C.; Schötzau, A.; Schorderet, D.F.; Valmaggia, C. Metabolic and functional changes in retinitis pigmentosa: Comparing retinal vessel oximetry to full-field electroretinography, electrooculogram and multifocal electroretinography. Acta Ophthalmol. 2016, 94, e231–e241. [Google Scholar] [CrossRef]
- Waizel, M.; Türksever, C.; Todorova, M.G. The effect of autoimmune retinopathy on retinal vessel oxygen saturation. Eye 2018, 32, 1455–1462. [Google Scholar] [CrossRef] [Green Version]
- Bojinova, R.I.; Schoderet, D.; Valmaggia, C.; Türksever, C.; Schoetzau, A.; Todorova, M.G. Higher retinal vessel oxygen saturation: Investigating its relationship with macular oedema in retinitis pigmentosa patients. Eye 2018, 32, 1209–1219. [Google Scholar] [CrossRef] [Green Version]
- Türksever, C.; Lópes Torres, L.T.; Valmaggia, C.; Todorova, M.G. Retinal Oxygenation in Inherited Diseases of the Retina. Genes 2021, 12, 272. [Google Scholar] [CrossRef]
- Kuroda, M.; Hirami, Y.; Hata, M.; Mandai, M.; Takahashi, M.; Kurimoto, Y. Intraretinal hyperreflective foci on spectral-domain optical coherence tomographic images of patients with retinitis pigmentosa. Clin. Ophthalmol. 2014, 24, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.H.; Yang, C.; Lai, Y.J.; Hsiao, C.K.; Hou, Y.C.; Yang, C.M.; Chen, T.C. Hyperreflective foci as important prognostic indicators of retinitis pigmentosa. Retina 2022, 42, 388–395. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Takahashi, A.; Nagaoka, T.; Ishibazawa, A.; Ishiko, S.; Yoshida, A. Retinal and choroidal hyperreflective foci on spectral-domain optical coherence tomographic images in a patient with retinitis pigmentosa accompanied by diabetic retinopathy. Am. J. Ophthalmol. Case Rep. 2016, 3, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Nagasaka, Y.; Ito, Y.; Ueno, S.; Terasaki, H. Number of Hyperreflective Foci in the Outer Retina Correlates with Inflammation and Photoreceptor Degeneration in Retinitis Pigmentosa. Ophthalmol. Retin. 2018, 2, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bocanegra, E.; Wozar, F.; Seitz, I.P.; Reichel, F.F.L.; Ochakovski, A.; Bucher, K.; Wilhelm, B.; Bartz-Schmidt, K.U.; Peters, T.; Fischer, M.D.; et al. Longitudinal Evaluation of Hyper-Reflective Foci in the Retina Following Subretinal Delivery of Adeno-Associated Virus in Non-Human Primates. Transl. Vis. Sci. Technol. 2021, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wu, D.; Xue, Y.; Wang, S.K.; Chung, M.J.; Ji, X.; Rana, P.; Zhao, S.R.; Mai, S.; Cepko, C.L. AAV cis-regulatory sequences are correlated with ocular toxicity. Proc. Natl. Acad. Sci. USA 2019, 116, 5785–5794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassisi, M.; Fan, W.; Shi, Y.; Lei, J.; Borrelli, E.; Ip, M.; Sadda, S.R. Quantity of Intraretinal Hyperreflective Foci in Patients With Intermediate Age-Related Macular Degeneration Correlates With 1-Year Progression. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3431–3439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, R.; Saurabh, K.; Shah, D.; Chowdhury, M.; Goel, S. Choroidal Hyperreflective Foci: A Novel Spectral Domain Optical Coherence Tomography Biomarker in Eyes With Diabetic Macular Edema. Asia Pac. J. Ophthalmol. 2019, 8, 314–318. [Google Scholar] [CrossRef]
- Rübsam, A.; Wernecke, L.; Rau, S.; Pohlmann, D.; Müller, B.; Zeitz, O.; Joussen, A.M. Behavior of SD-OCT Detectable Hyperreflective Foci in Diabetic Macular Edema Patients after Therapy with Anti-VEGF Agents and Dexamethasone Implants. J. Diabetes Res. 2021, 2021, 8820216. [Google Scholar] [CrossRef]
- Couturier, A.; Mane, V.; Lavia, C.A.; Tadayoni, R. Hyperreflective cystoid spaces in diabetic macular oedema: Prevalence and clinical implications. Br. J. Ophthalmol. 2022, 106, 540–546. [Google Scholar] [CrossRef]
- Mueller, S.; Gunnemann, F.; Rothaus, K.; Book, M.; Faatz, H.; Bird, A.; Pauleikhoff, D. Incidence and phenotypical variation of outer retina-associated hyperreflectivity in macular telangiectasia type 2. Br. J. Ophthalmol. 2021, 105, 573–576. [Google Scholar] [CrossRef]
- Yang, Q.; Wei, W.; Shi, X.; Yang, L. Successful use of intravitreal ranibizumab injection and combined treatment in the management of Coats’ disease. Acta Ophthalmol. 2016, 94, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Mo, B.; Zhou, H.; Jiao, X.; Zhang, F. Evaluation of hyperreflective foci as a prognostic factor of visual outcome in retinal vein occlusion. Int. J. Ophthalmol. 2017, 10, 605–612. [Google Scholar]
- Battaglia Parodi, M.; Sacconi, R.; Romano, F.; Bandello, F. Hyperreflective foci in Stargardt disease: 1-year follow-up. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hanumunthadu, D.; Rasheed, M.; Goud, A.; Gupta, A.; Vupparaboina, K.K.; Chhablani, J. Choroidal hyper-reflective foci and vascularity in retinal dystrophy. Indian J. Ophthalmol. 2020, 68, 130–133. [Google Scholar] [PubMed]
- Bolz, M.; Schmidt-Erfurth, U.; Deak, G.; Mylonas, G.; Kriechbaum, K.; Scholda, C. Diabetic Retinopathy Research Group Vienna. Optical coherence tomographic hyperreflective foci: A morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology 2009, 116, 914–920. [Google Scholar] [PubMed]
- Fragiotta, S.; Abdolrahimzadeh, S.; Dolz-Marco, R.; Sakurada, Y.; Gal-Or, O.; Scuderi, G. Significance of Hyperreflective Foci as an Optical Coherence Tomography Biomarker in Retinal Diseases: Characterization and Clinical Implications. J. Ophthalmol. 2021, 2021, 6096017. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Waldstein, S. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog. Retin. Eye Res. 2016, 50, 1–24. [Google Scholar] [CrossRef]
- Fariss, R.N.; Li, Z.Y.; Milam, A.H. Abnormalities in rod photoreceptors, amacrine cells, and horizontal cells in human retinas with retinitis pigmentosa. Am. J. Ophthalmol. 2000, 129, 215–223. [Google Scholar] [CrossRef]
- Milam, A.H.; Li, Z.Y.; Cideciyan, A.V.; Jacobson, S.G. Clinicopathologic effects of the Q64ter rhodopsin mutation in retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 1996, 37, 753–765. [Google Scholar]
- Milam, A.H.; Li, Z.Y.; Fariss, R.N. Histopathology of the human retina in retinitis pigmentosa. Prog. Retin. Eye Res. 1998, 17, 175–205. [Google Scholar]
- Merin, S.; Auerbach, E. Retinitis pigmentosa. Surv. Ophthalmol. 1976, 20, 303–346. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Ma, Y.; Kawasaki, R.; Dobson, L.P.; Ruddle, J.B.; Kearns, L.S.; Wong, T.Y.; Mackey, D.A. Quantitative analysis of retinal vessel attenuation in eyes with retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4306–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eysteinsson, T.; Hardarson, S.; Bragason, D.; Stefánsson, E. Retinal vessel oxygen saturation and vessel diameter in retinitis pigmentosa. Acta Ophthalmol. 2014, 92, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Vanderkooi, J.M.; Erecinska, M.; Silver, I.A. Oxygen in mammalian tissue: Methods of measurement and affinities of various reactions. Am. J. Physiol. 1991, 260, C1131–C1150. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Y.; Cringle, S.J.; Su, E.N.; Yu, P.K. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3999–4006. [Google Scholar]
- Yu, D.Y.; Cringle, S.J.; Valter, K.; Walsh, N.; Lee, D.; Stone, J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Y.; Cringle, S.J. Retinal degeneration and local oxygen metabolism. Exp. Eye Res. 2005, 80, 745–751. [Google Scholar] [CrossRef]
- Padnick-Silver, L.; Kang Derwent, J.J.; Giuliano, E.; Narfström, K.; Linsenmeier, R.A. Retinal oxygenation and oxygen metabolism in Abyssinian cats with a hereditary retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3683–3689. [Google Scholar] [CrossRef]
- Usui, S.; Overson, B.C.; Lee, S.Y.; Jo, Y.J.; Yoshida, T.; Miki, A.; Miki, K.; Iwase, T.; Lu, L.; Campochiaro, P.A. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J. Neurochem. 2009, 110, 1028–1037. [Google Scholar] [CrossRef] [Green Version]
- Wirtschafter, J. Optic nerve axons and acquired alterations in the appearance of the optic disc. Trans. Am. Ophthalmol. Soc. 1983, 81, 1034–1091. [Google Scholar]
- Ayton, L.N.; Guymer, R.; Luu, C.D. Choroidal thickness profiles in retinitis pigmentosa. Clin. Exp. Ophthalmol. 2013, 41, 396–403. [Google Scholar]
- Tan, R.; Agrawal, R.; Taduru, S.; Gupta, A.; Vupparaboina, K.; Chhablani, J. Choroidal Vascularity Index in Retinitis Pigmentosa: An OCT Study. Ophthalmic Surg. Lasers Imaging Retin. 2018, 49, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Bordato, A.; Romano, F.; Aragona, E.; Grazioli, A.; Bandello, F.; Battaglia Parodi, M. Choroidal Patterns in Retinitis Pigmentosa: Correlation with Visual Acuity and Disease Progression. Transl. Vis. Sci. Technol. 2020, 9, 17. [Google Scholar] [PubMed]
- Egawa, M.; Mitamura, Y.; Niki, M.; Sano, H.; Miura, G.; Chiba, A.; Yamamoto, S.; Sonoda, S.; Sakamoto, T. Correlations between choroidal structures and visual functions in eyes with retinitis pigmentosa. Retina 2019, 39, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Marc, R.E.; Jones, B.W. Retinal remodeling in inherited photoreceptor degenerations. Mol. Neurobiol. 2003, 28, 139–147. [Google Scholar] [CrossRef]
- Marc, R.E.; Jones, B.W.; Watt, C.B.; Strettoi, E. Neural remodeling in retinal degeneration. Prog. Retin. Eye Res. 2003, 22, 607–655. [Google Scholar] [CrossRef]
- Cottet, S.; Schorderet, D.F. Mechanisms of apoptosis in retinitis pigmentosa. Curr. Mol. Med. 2009, 9, 375–383. [Google Scholar] [CrossRef]
- Grunwald, J.E.; Maguire, A.M.; Dupont, J. Retinal hemodynamics in retinitis pigmentosa. Am. J. Ophthalmol. 1996, 122, 502–508. [Google Scholar] [CrossRef]
- Beutelspacher, S.C.; Serbecic, N.; Barash, H.; Burgansky-Eliash, Z.; Grinvald, A.; Krastel, H.; Jonas, J.B. Retinal blood flow velocity measured by retinal function imaging in retinitis pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1855–1858. [Google Scholar] [CrossRef]
- Konieczka, K.; Bojinova, R.; Valmaggia, C.; Schorderet, D.F.; Todorova, M.G.; Medscape. Preserved functional and structural integrity of the papillomacular area correlates with better visual acuity in retinitis pigmentosa. Eye 2016, 30, 1310–1323. [Google Scholar] [CrossRef]
- Moon, C.H.; Park, T.; Ohn, Y.H. Association between multifocal electroretinograms, optical coherence tomography and central visual sensitivity in advanced retinitis pigmentosa. Doc. Ophthalmol. 2012, 125, 113–122. [Google Scholar] [CrossRef]
- Yoon, C.K.; Yu, H.G. The Structure-Function Relationship between Macular Morphology and Visual Function Analyzed by Optical Coherence Tomography in Retinitis Pigmentosa. J. Ophthalmol. 2013, 2013, 821460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fishman, G.A.; Fishman, M.; Maggiano, J. Macular lesions associated with retinitis pigmentosa. Arch. Ophthalmol. 1977, 95, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Ekart, A.; Gherghel, D. Ageing effect on flicker-induced diameter changes in retinal microvessels of healthy individuals. Acta Ophthalmol. 2016, 94, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Waizel, M.; Türksever, C.; Todorova, M.G. Normative values of retinal vessel oximetry in healthy children against adults. Acta Ophthalmol. 2018, 96, e828–e834. [Google Scholar] [CrossRef]





| Demographic Characteristics | Retinitis Pigmentosa Patients | Controls |
|---|---|---|
| Number of Subjects (eyes) | 42 (84) | 24 (48) |
| Mean Age [± SD] | 49.76 [14.94] | 42.88 [16.32] |
| Sex: ♀/♂ | 20/22 | 14/8 |
| Parameters | Groups | Mean | ±SD | p-Values between Controls and RP Patients |
|---|---|---|---|---|
| BCVA (Snellen chart) | Controls RP | 0.92 0.69 | 0.23 0.36 | <0.001 |
| HRF-ER, number | Controls RP | 36.83 100.45 | 14.14 49.77 | <0.001 |
| HRF-ONL, number | Controls RP | 8.72 31.4 | 6.97 20.68 | <0.001 |
| EZ length, µm | Controls RP | 5000 2511.28 | 0 1837.86 | <0.001 |
| D-A, µm | Controls RP | 116.77 92.79 | 9.70 18.5 | <0.001 |
| D-V, µm | Controls RP | 158.09 123.31 | 16.26 22.48 | <0.001 |
| A-SO2, % | Controls RP | 98.60 101.37 | 2.13 5.85 | 0.049 |
| V-SO2,% | Controls RP | 60.99 65.73 | 3.12 5.87 | 0.001 |
| A-V SO2, % | Controls RP | 37.61 35.64 | 2.87 7.12 | 0.198 |
| Variables | Predictors | |||||
|---|---|---|---|---|---|---|
| HRF-ER | HRF-ONL | |||||
| Both RP and Controls | RP | Controls | Both Groups | RP | Controls | |
| IS/OS length [μm] RP-ME RP-no-ME | <0.001 | <0.001 <0.001 <0.001 | <0.001 | 0.002 <0.001 0.005 | ||
| BCVA [Snellen chart] RP-ME RP-no-ME | <0.001 | 0.001 <0.001 | 0.044 | 0.011 <0.001 | ||
| HRF-ONL [count] RP-ME RP-no-ME | <0.001 | <0.001 <0.001 | <0.001 | |||
| HRF-ER [count] RP-ME RP-no-ME | <0.001 <0.001 | <0.001 | <0.001 | |||
| D-A [μm] RP-ME RP-no-ME | 0.018 | 0.002 0.008 | ||||
| D-V [μm] RP-ME RP-no-ME | ||||||
| A-SO2 [%] RP-ME RP-no-ME | 0.002 | 0.006 | 0.018 | 0.037 | ||
| V-SO2 [%] RP-ME RP-no-ME | ||||||
| A-V SO2 [%] RP-ME RP-no-ME | 0.005 | 0.022 0.022 | 0.014 | 0.048 0.048 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diem, C.; Türksever, C.; Todorova, M.G. The Presence of Hyperreflective Foci Reflects Vascular, Morphologic and Metabolic Alterations in Retinitis Pigmentosa. Genes 2022, 13, 2034. https://doi.org/10.3390/genes13112034
Diem C, Türksever C, Todorova MG. The Presence of Hyperreflective Foci Reflects Vascular, Morphologic and Metabolic Alterations in Retinitis Pigmentosa. Genes. 2022; 13(11):2034. https://doi.org/10.3390/genes13112034
Chicago/Turabian StyleDiem, Clemens, Cengiz Türksever, and Margarita G. Todorova. 2022. "The Presence of Hyperreflective Foci Reflects Vascular, Morphologic and Metabolic Alterations in Retinitis Pigmentosa" Genes 13, no. 11: 2034. https://doi.org/10.3390/genes13112034