PPAR-Mediated Toxicology and Applied Pharmacology
Abstract
:1. Introduction
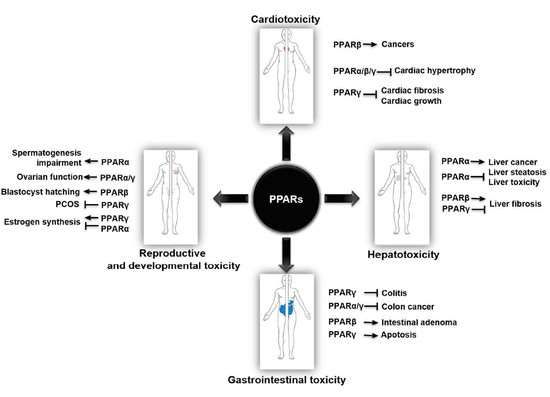
2. PPARs in Cardiotoxicity
3. PPARs in Hepatotoxicity
4. PPARs in Gastrointestinal Toxicity
5. PPARs in Reproductive and Developmental Toxicity
6. Other Systemic Toxicity and Protective Effects Mediated by PPARs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grygiel-Gorniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [Green Version]
- Lagana, A.S.; Vitale, S.G.; Nigro, A.; Sofo, V.; Salmeri, F.M.; Rossetti, P.; Rapisarda, A.M.; La Vignera, S.; Condorelli, R.A.; Rizzo, G.; et al. Pleiotropic Actions of Peroxisome Proliferator-Activated Receptors (PPARs) in Dysregulated Metabolic Homeostasis, Inflammation and Cancer: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2016, 17, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, F.; Xu, P.; Zhai, Y. The Opportunities and Challenges of Peroxisome Proliferator-Activated Receptors Ligands in Clinical Drug Discovery and Development. Int. J. Mol. Sci. 2018, 19, 2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, J.; Wagner, J.A. Physiological and therapeutic roles of peroxisome proliferator-activated receptors. Diabetes Technol. Ther. 2002, 4, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Amber-Vitos, O.; Chaturvedi, N.; Nachliel, E.; Gutman, M.; Tsfadia, Y. The effect of regulating molecules on the structure of the PPAR-RXR complex. Biochim. Biophys. Acta 2016, 1861, 1852–1863. [Google Scholar] [CrossRef]
- Peraza, M.A.; Burdick, A.D.; Marin, H.E.; Gonzalez, F.J.; Peters, J.M. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR). Toxicol. Sci. 2006, 90, 269–295. [Google Scholar] [CrossRef] [Green Version]
- Corrales, P.; Vidal-Puig, A.; Medina-Gomez, G. PPARs and Metabolic Disorders Associated with Challenged Adipose Tissue Plasticity. Int. J. Mol. Sci. 2018, 19, 2124. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as Nuclear Receptors for Nutrient and Energy Metabolism. Molecules 2019, 24, 2545. [Google Scholar] [CrossRef] [Green Version]
- Boeckmans, J.; Natale, A.; Rombaut, M.; Buyl, K.; Rogiers, V.; De Kock, J.; Vanhaecke, T.; Rodrigues, M.R. Anti-NASH Drug Development Hitches a Lift on PPAR Agonism. Cells 2019, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Funahashi, T.; Yamashita, S.; Nishida, M.; Nishida, Y.; Takahashi, M.; Hotta, K.; Kuriyama, H.; Kihara, S.; Ohuchi, N.; et al. Thiazolidinedione derivative improves fat distribution and multiple risk factors in subjects with visceral fat accumulation--double-blind placebo-controlled trial. Diabetes Res. Clin. Pract. 2001, 54, 181–190. [Google Scholar] [CrossRef]
- Watkins, P.B.; Whitcomb, R.W. Hepatic dysfunction associated with troglitazone. N. Engl. J. Med. 1998, 338, 916–917. [Google Scholar] [CrossRef] [PubMed]
- Rubenstrunk, A.; Hanf, R.; Hum, D.W.; Fruchart, J.C.; Staels, B. Safety issues and prospects for future generations of PPAR modulators. Biochim. Biophys. Acta 2007, 1771, 1065–1081. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wu, X.; Huang, Q.; Liao, Y.; Liu, L.; Qiu, L.; Shen, H.; Dong, S. PFOS elicits transcriptional responses of the ER, AHR and PPAR pathways in Oryzias melastigma in a stage-specific manner. Aquat. Toxicol. 2012, 106, 9–19. [Google Scholar] [CrossRef]
- Huang, Q.; Fang, C.; Wu, X.; Fan, J.; Dong, S. Perfluorooctane sulfonate impairs the cardiac development of a marine medaka (Oryzias melastigma). Aquat. Toxicol. 2011, 105, 71–77. [Google Scholar] [CrossRef]
- Zhao, M.; Jiang, Q.; Geng, M.; Zhu, L.; Xia, Y.; Khanal, A.; Wang, C. The role of PPAR alpha in perfluorooctanoic acid induced developmental cardiotoxicity and l-carnitine mediated protection-Results of in ovo gene silencing. Environ. Toxicol. Pharmacol. 2017, 56, 136–144. [Google Scholar] [CrossRef]
- Posnack, N.G.; Swift, L.M.; Kay, M.W.; Lee, N.H.; Sarvazyan, N. Phthalate exposure changes the metabolic profile of cardiac muscle cells. Environ. Health Perspect. 2012, 120, 1243–1251. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, W.; Ren, J.; Li, W.; Zhou, L.; Cui, Y.; Chen, H.; Yu, W.; Zhuang, X.; Zhang, Z.; et al. Metabonomics Indicates Inhibition of Fatty Acid Synthesis, beta-Oxidation, and Tricarboxylic Acid Cycle in Triclocarban-Induced Cardiac Metabolic Alterations in Male Mice. J. Agric. Food Chem. 2018, 66, 1533–1542. [Google Scholar] [CrossRef]
- Ebenstein, A.; Fan, M.; Greenstone, M.; He, G.; Zhou, M. New evidence on the impact of sustained exposure to air pollution on life expectancy from China’s Huai River Policy. Proc. Natl. Acad. Sci. USA 2017, 114, 10384–10389. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Xue, B.; Ahmed, R.Z.; Ding, G.; Li, Z. Fine particles cause the abnormality of cardiac ATP levels via PPARa-mediated utilization of fatty acid and glucose using in vivo and in vitro models. Environ. Pollut. 2019, 249, 286–294. [Google Scholar] [CrossRef]
- Luptak, I.; Balschi, J.A.; Xing, Y.; Leone, T.C.; Kelly, D.P.; Tian, R. Decreased contractile and metabolic reserve in peroxisome proliferator-activated receptor-alpha-null hearts can be rescued by increasing glucose transport and utilization. Circulation 2005, 112, 2339–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, S.Z.; Ivashchenko, C.Y.; Russell, M.W.; Milstone, D.S.; Mortensen, R.M. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-γ both induce cardiac hypertrophy in mice. Circ. Res. 2005, 97, 372–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.I.; Kim, W.S.; Kim, D.H.; Kang, J.S. Histopathological Evaluation of Heart Toxicity of a Novel Selective PPAR-γ Agonists CKD-501 in db/db Mice. Biomol. Ther. 2013, 21, 84–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Tao, H.; Xiong, H.; Duan, S.Z.; McGowan, F.X., Jr.; Mortensen, R.M.; Balschi, J.A. Rosiglitazone causes cardiotoxicity via peroxisome proliferator-activated receptor gamma-independent mitochondrial oxidative stress in mouse hearts. Toxicol. Sci. 2014, 138, 468–481. [Google Scholar] [CrossRef] [Green Version]
- Planavila, A.; Laguna, J.C.; Vazquez-Carrera, M. Atorvastatin improves peroxisome proliferator-activated receptor signaling in cardiac hypertrophy by preventing nuclear factor-kappa B activation. Biochim. Biophys. Acta 2005, 1687, 76–83. [Google Scholar] [CrossRef]
- Malekinejad, M.; Masoumi Verki, M.; Khoramjouy, M.; Alenabi, A.; Hallaj-Salahipour, M.; Malekinejad, H. Cardioprotective Effects of Atorvastatin Are Mediated Through PPARgamma in Paraquat-Exposed Rats. J. Cardiovasc. Pharmacol. 2019, 74, 400–408. [Google Scholar] [CrossRef]
- Bhargava, P.; Verma, V.K.; Malik, S.; Khan, S.I.; Bhatia, J.; Arya, D.S. Hesperidin regresses cardiac hypertrophy by virtue of PPAR-γ agonistic, anti-inflammatory, antiapoptotic, and antioxidant properties. J. Biochem. Mol. Toxicol. 2019, 33, e22283. [Google Scholar] [CrossRef]
- Ma, Z.G.; Yuan, Y.P.; Zhang, X.; Xu, S.C.; Wang, S.S.; Tang, Q.Z. Piperine Attenuates Pathological Cardiac Fibrosis via PPAR-γ/AKT Pathways. EBioMedicine 2017, 18, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Asakawa, M.; Takano, H.; Nagai, T.; Uozumi, H.; Hasegawa, H.; Kubota, N.; Saito, T.; Masuda, Y.; Kadowaki, T.; Komuro, I. Peroxisome proliferator-activated receptor gamma plays a critical role in inhibition of cardiac hypertrophy in vitro and in vivo. Circulation 2002, 105, 1240–1246. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, Y.; Long, B.; Qian, J.; Perez-Polo, J.R.; Ye, Y. Pioglitazone limits myocardial infarct size, activates Akt, and upregulates cPLA2 and COX-2 in a PPAR-γ-independent manner. Basic Res. Cardiol. 2011, 106, 431–446. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, J.; Xu, X.; Jing, Y.; Tu, L.; Li, X.; Chen, C.; Cianflone, K.; Wang, P.; Dackor, R.T.; et al. Epoxyeicosatrienoic acids protect rat hearts against tumor necrosis factor-alpha-induced injury. J. Lipid Res. 2012, 53, 456–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalliora, C.; Kyriazis, I.D.; Oka, S.I.; Lieu, M.J.; Yue, Y.; Area-Gomez, E.; Pol, C.J.; Tian, Y.; Mizushima, W.; Chin, A.; et al. Dual peroxisome-proliferator-activated-receptor-alpha/gamma activation inhibits SIRT1-PGC1alpha axis and causes cardiac dysfunction. JCI Insight 2019, 5, 129556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engle, S.K.; Solter, P.F.; Credille, K.M.; Bull, C.M.; Adams, S.; Berna, M.J.; Schultze, A.E.; Rothstein, E.C.; Cockman, M.D.; Pritt, M.L.; et al. Detection of left ventricular hypertrophy in rats administered a peroxisome proliferator-activated receptor alpha/gamma dual agonist using natriuretic peptides and imaging. Toxicol. Sci. 2010, 114, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Chen, H.; Birnbaum, Y.; Nanhwan, M.K.; Bajaj, M.; Ye, Y.; Qian, J. Aleglitazar, a dual peroxisome proliferator-activated receptor-alpha and -gamma agonist, protects cardiomyocytes against the adverse effects of hyperglycaemia. Diab. Vasc. Dis. Res. 2017, 14, 152–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khuchua, Z.; Glukhov, A.I.; Strauss, A.W.; Javadov, S. Elucidating the Beneficial Role of PPAR Agonists in Cardiac Diseases. Int. J. Mol. Sci. 2018, 19, 3464. [Google Scholar] [CrossRef] [Green Version]
- Samokhvalov, V.; Zlobine, I.; Jamieson, K.L.; Jurasz, P.; Chen, C.; Lee, K.S.; Hammock, B.D.; Seubert, J.M. PPARdelta signaling mediates the cytotoxicity of DHA in H9c2 cells. Toxicol. Lett. 2015, 232, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.C.; Chen, L.J.; Cheng, J.T. Doxorubicin-Induced Cardiac Toxicity Is Mediated by Lowering of Peroxisome Proliferator-Activated Receptor delta Expression in Rats. PPAR Res. 2013, 2013, 456042. [Google Scholar] [CrossRef] [Green Version]
- Wagner, K.D.; Du, S.; Martin, L.; Leccia, N.; Michiels, J.F.; Wagner, N. Vascular PPARbeta/delta Promotes Tumor Angiogenesis and Progression. Cells 2019, 8, 1623. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Nakajima, T. PPARalpha- and DEHP-Induced Cancers. PPAR Res. 2008, 2008, 759716. [Google Scholar] [CrossRef] [Green Version]
- Casals-Casas, C.; Feige, J.N.; Desvergne, B. Interference of pollutants with PPARs: Endocrine disruption meets metabolism. Int. J. Obes. (Lond.) 2008, 32, S53–S61. [Google Scholar] [CrossRef] [Green Version]
- Stahlhut, R.W.; van Wijngaarden, E.; Dye, T.D.; Cook, S.; Swan, S.H. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insuslin resistance in adult U.S. males. Environ. Health Perspect. 2007, 115, 876–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, X.; Niu, L.; Li, Q. Proteomics Analysis Reveals an Important Role for the PPAR Signaling Pathway in DBDCT-Induced Hepatotoxicity Mechanisms. Molecules 2017, 22, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, X.; Li, Y.; Mai, J.; Ji, X.; Li, Q. Effect of Dibutyltin Dilaurate on Triglyceride Metabolism through the Inhibition of the mTOR Pathway in Human HL7702 Liver Cells. Molecules 2018, 23, 1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upham, J.; Acott, P.D.; O’Regan, P.; Sinal, C.J.; Crocker, J.F.; Geldenhuys, L.; Murphy, M.G. The pesticide adjuvant, Toximul, alters hepatic metabolism through effects on downstream targets of PPARalpha. Biochim. Biophys. Acta 2007, 1772, 1057–1064. [Google Scholar] [CrossRef] [Green Version]
- Grun, F.; Watanabe, H.; Zamanian, Z.; Maeda, L.; Arima, K.; Cubacha, R.; Gardiner, D.M.; Kanno, J.; Iguchi, T.; Blumberg, B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 2006, 20, 2141–2155. [Google Scholar] [CrossRef]
- Jeng, L.B.; Velmurugan, B.K.; Hsu, H.H.; Wen, S.Y.; Shen, C.Y.; Lin, C.H.; Lin, Y.M.; Chen, R.J.; Kuo, W.W.; Huang, C.Y. Fenofibrate induced PPAR alpha expression was attenuated by oestrogen receptor alpha overexpression in Hep3B cells. Environ. Toxicol. 2018, 33, 234–247. [Google Scholar] [CrossRef]
- Nakajima, T.; Kamijo, Y.; Tanaka, N.; Sugiyama, E.; Tanaka, E.; Kiyosawa, K.; Fukushima, Y.; Peters, J.M.; Gonzalez, F.J.; Aoyama, T. Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology 2004, 40, 972–980. [Google Scholar] [CrossRef]
- Anderson, S.P.; Howroyd, P.; Liu, J.; Qian, X.; Bahnemann, R.; Swanson, C.; Kwak, M.K.; Kensler, T.W.; Corton, J.C. The transcriptional response to a peroxisome proliferator-activated receptor alpha agonist includes increased expression of proteome maintenance genes. J. Biol. Chem. 2004, 279, 52390–52398. [Google Scholar] [CrossRef] [Green Version]
- Shankar, K.; Vaidya, V.S.; Corton, J.C.; Bucci, T.J.; Liu, J.; Waalkes, M.P.; Mehendale, H.M. Activation of PPAR-alpha in streptozotocin-induced diabetes is essential for resistance against acetaminophen toxicity. FASEB J. 2003, 17, 1748–1750. [Google Scholar] [CrossRef]
- You, M.; Crabb, D.W. Recent advances in alcoholic liver disease II. Minireview: Molecular mechanisms of alcoholic fatty liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1–G6. [Google Scholar] [CrossRef] [Green Version]
- Kon, K.; Ikejima, K.; Hirose, M.; Yoshikawa, M.; Enomoto, N.; Kitamura, T.; Takei, Y.; Sato, N. Pioglitazone prevents early-phase hepatic fibrogenesis caused by carbon tetrachloride. Biochem. Bioph. Res. Commun. 2002, 291, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, S.M.; Lee, G.H.; Jin, S.W.; Lee, H.S.; Chung, Y.C.; Jeong, H.G. Platyconic Acid A, Platycodi Radix-Derived Saponin, Suppresses TGF-1-induced Activation of Hepatic Stellate Cells via Blocking SMAD and Activating the PPAR Signaling Pathway. Cells 2019, 8, 1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellemans, K.; Michalik, L.; Dittie, A.; Knorr, A.; Rombouts, K.; De Jong, J.; Heirman, C.; Quartier, E.; Schuit, F.; Wahli, W.; et al. Peroxisome proliferator-activated receptor-beta signaling contributes to enhanced proliferation of hepatic stellate cells. Gastroenterology 2003, 124, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Hays, T.; Rusyn, I.; Burns, A.M.; Kennett, M.J.; Ward, J.M.; Gonzalez, F.J.; Peters, J.M. Role of peroxisome proliferator-activated receptor-alpha (PPARalpha) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis 2005, 26, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Corton, J.C.; Peters, J.M.; Klaunig, J.E. The PPARalpha-dependent rodent liver tumor response is not relevant to humans: Addressing misconceptions. Arch. Toxicol. 2018, 92, 83–119. [Google Scholar] [CrossRef] [Green Version]
- Reddy, J.K.; Chu, R. Peroxisome proliferator-induced pleiotropic responses: Pursuit of a phenomenon. Ann. N. Y. Acad. Sci. 1996, 804, 176–201. [Google Scholar] [CrossRef]
- Werle-Schneider, G.; Wolfelschneider, A.; von Brevern, M.C.; Scheel, J.; Storck, T.; Muller, D.; Glockner, R.; Bartsch, H.; Bartelmann, M. Gene expression profiles in rat liver slices exposed to hepatocarcinogenic enzyme inducers, peroxisome proliferators, and 17alpha-ethinylestradiol. Int. J. Toxicol. 2006, 25, 379–395. [Google Scholar] [CrossRef]
- Corton, J.C.; Lapinskas, P.J.; Gonzalez, F.J. Central role of PPARalpha in the mechanism of action of hepatocarcinogenic peroxisome proliferators. Mutat. Res. 2000, 448, 139–151. [Google Scholar] [CrossRef]
- Michalik, L.; Desvergne, B.; Wahli, W. Peroxisome-proliferator-activated receptors and cancers: Complex stories. Nat. Rev. Cancer 2004, 4, 61–70. [Google Scholar] [CrossRef]
- Boitier, E.; Gautier, J.C.; Roberts, R. Advances in understanding the regulation of apoptosis and mitosis by peroxisome-proliferator activated receptors in pre-clinical models: Relevance for human health and disease. Comp. Hepatol. 2003, 2, 3. [Google Scholar] [CrossRef]
- Clark, R.B.; Bishop-Bailey, D.; Estrada-Hernandez, T.; Hla, T.; Puddington, L.; Padula, S.J. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. J. Immunol. 2000, 164, 1364–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Luo, H.; Wang, N.; Li, J.; Xu, S.; Chen, K.; Feng, J.; Wu, L.; Li, S.; Liu, T.; et al. Portulaca Extract Attenuates Development of Dextran Sulfate Sodium Induced Colitis in Mice through Activation of PPARgamma. PPAR Res. 2018, 2018, 6079101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccardi, L.; Mazzon, E.; Bruscoli, S.; Esposito, E.; Crisafulli, C.; Di Paola, R.; Caminiti, R.; Riccardi, C.; Cuzzocrea, S. Peroxisome proliferator-activated receptor-alpha modulates the anti-inflammatory effect of glucocorticoids in a model of inflammatory bowel disease in mice. Shock 2009, 31, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, H.; Xu, L.; Liao, Q.; Wan, S.; Yu, Z.; Lin, D.; Zhang, B.; Lv, Z.; Wu, Z.; et al. rSj16 Protects against DSS-Induced Colitis by Inhibiting the PPAR-alpha Signaling Pathway. Theranostics 2017, 7, 3446–3460. [Google Scholar] [CrossRef] [PubMed]
- Auboeuf, D.; Rieusset, J.; Fajas, L.; Vallier, P.; Frering, V.; Riou, J.P.; Staels, B.; Auwerx, J.; Laville, M.; Vidal, H. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: No alteration in adipose tissue of obese and NIDDM patients. Diabetes 1997, 46, 1319–1327. [Google Scholar] [CrossRef]
- Zieleniak, A.; Wojcik, M.; Wozniak, L.A. Structure and physiological functions of the human peroxisome proliferator-activated receptor gamma. Arch. Immunol. Ther. Exp. 2008, 56, 331–345. [Google Scholar] [CrossRef]
- Da Silva, S.; Keita, A.V.; Mohlin, S.; Pahlman, S.; Theodorou, V.; Pahlman, I.; Mattson, J.P.; Soderholm, J.D. A Novel Topical PPARgamma Agonist Induces PPARgamma Activity in Ulcerative Colitis Mucosa and Prevents and Reverses Inflammation in Induced Colitis Models. Inflamm. Bowel Dis. 2018, 24, 792–805. [Google Scholar] [CrossRef]
- Desreumaux, P.; Dubuquoy, L.; Nutten, S.; Peuchmaur, M.; Englaro, W.; Schoonjans, K.; Derijard, B.; Desvergne, B.; Wahli, W.; Chambon, P.; et al. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J. Exp. Med. 2001, 193, 827–838. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, A.; Wada, K.; Miki, H.; Kubota, N.; Nakajima, N.; Terauchi, Y.; Ohnishi, S.; Saubermann, L.J.; Kadowaki, T.; Blumberg, R.S.; et al. Endogenous PPAR gamma mediates anti-inflammatory activity in murine ischemia-reperfusion injury. Gastroenterology 2001, 120, 460–469. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.; Sottili, M.; Gerini, C.; Desideri, I.; Bastida, C.; Pallotta, S.; Castiglione, F.; Bonomo, P.; Meattini, I.; Greto, D.; et al. A PPAR-γ agonist protects from radiation-induced intestinal toxicity. United Eur. Gastroenterol J. 2017, 5, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Linard, C.; Souidi, M. PPARs in Irradiation-Induced Gastrointestinal Toxicity. PPAR Res. 2010, 2010, 528327. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Peng, Z.; Luo, S.; Zhang, S.; Li, B.; Zhou, C.; Fan, H. Aesculin protects against DSS-Induced colitis though activating PPARgamma and inhibiting NF-small ka, CyrillicB pathway. Eur. J. Pharmacol. 2019, 857, 172453. [Google Scholar] [CrossRef] [PubMed]
- Linard, C.; Gremy, O.; Benderitter, M. Reduction of peroxisome proliferation-activated receptor gamma expression by gamma-irradiation as a mechanism contributing to inflammatory response in rat colon: Modulation by the 5-aminosalicylic acid agonist. J. Pharmacol. Exp. Ther. 2008, 324, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xie, C.; Brocker, C.N.; Fan, J.; Wu, X.; Feng, L.; Wang, Q.; Zhao, J.; Lu, D.; Tandon, M.; et al. Intestinal PPARalpha Protects Against Colon Carcinogenesis via Regulation of Methyltransferases DNMT1 and PRMT6. Gastroenterology 2019, 157, 744–759 e4. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Curtin, K.; Wolff, R.; Ma, K.N.; Sweeney, C.; Murtaugh, M.; Potter, J.D.; Levin, T.R.; Samowitz, W. PPARgamma and colon and rectal cancer: Associations with specific tumor mutations, aspirin, ibuprofen and insulin-related genes (United States). Cancer Causes Control. 2006, 17, 239–249. [Google Scholar] [CrossRef]
- Sabatino, L.; Ziccardi, P.; Cerchia, C.; Muccillo, L.; Piemontese, L.; Loiodice, F.; Colantuoni, V.; Lupo, A.; Lavecchia, A. Chiral phenoxyacetic acid analogues inhibit colon cancer cell proliferation acting as PPARgamma partial agonists. Sci. Rep. 2019, 9, 5434. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.L.; Frucht, H. Activation of the PPAR pathway induces apoptosis and COX-2 inhibition in HT-29 human colon cancer cells. Carcinogenesis 2001, 22, 1379–1383. [Google Scholar] [CrossRef] [Green Version]
- Sarraf, P.; Mueller, E.; Jones, D.; King, F.J.; DeAngelo, D.J.; Partridge, J.B.; Holden, S.A.; Chen, L.B.; Singer, S.; Fletcher, C.; et al. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat. Med. 1998, 4, 1046–1052. [Google Scholar] [CrossRef]
- Lian, B.; Ren, Y.; Zhang, H.; Lin, T.; Wang, Y. An adenosine derivative (IFC-305) reduced the risk of radiation-induced intestinal toxicity in the treatment of colon cancer by suppressing the methylation of PPAR-r promoter. Biomed. Pharmacother. 2019, 118, 109202. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, S.; Mana, M.D.; Roper, J.; Kedrin, D.; Saadatpour, A.; Hong, S.J.; Bauer-Rowe, K.E.; Xifaras, M.E.; Akkad, A.; Arias, E.; et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016, 531, 53–58. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Yuan, S.; Shi, J.; Hou, Y. PPARdelta signaling regulates colorectal cancer. Curr. Pharm. Des. 2015, 21, 2956–2959. [Google Scholar] [CrossRef] [PubMed]
- Vitti, M.; Di Emidio, G.; Di Carlo, M.; Carta, G.; Antonosante, A.; Artini, P.G.; Cimini, A.; Tatone, C.; Benedetti, E. Peroxisome Proliferator-Activated Receptors in Female Reproduction and Fertility. PPAR Res. 2016, 2016, 4612306. [Google Scholar] [CrossRef]
- Froment, P.; Gizard, F.; Defever, D.; Staels, B.; Dupont, J.; Monget, P. Peroxisome proliferator-activated receptors in reproductive tissues: From gametogenesis to parturition. J. Endocrinol. 2006, 189, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Chen, Q. Mediating Roles of PPARs in the Effects of Environmental Chemicals on Sex Steroids. PPAR Res. 2017, 2017, 3203161. [Google Scholar] [CrossRef]
- Ziaei, S.; Halaby, R. Immunosuppressive, anti-inflammatory and anti-cancer properties of triptolide: A mini review. Avicenna J. Phytomed. 2016, 6, 149–164. [Google Scholar]
- Cheng, Y.; Chen, G.; Wang, L.; Kong, J.; Pan, J.; Xi, Y.; Shen, F.; Huang, Z. Triptolide-induced mitochondrial damage dysregulates fatty acid metabolism in mouse sertoli cells. Toxicol. Lett. 2018, 292, 136–150. [Google Scholar] [CrossRef]
- Ma, B.; Qi, H.; Li, J.; Xu, H.; Chi, B.; Zhu, J.; Yu, L.; An, G.; Zhang, Q. Triptolide disrupts fatty acids and peroxisome proliferator-activated receptor (PPAR) levels in male mice testes followed by testicular injury: A GC-MS based metabolomics study. Toxicology 2015, 336, 84–95. [Google Scholar] [CrossRef]
- Philips, E.M.; Jaddoe, V.W.V.; Trasande, L. Effects of early exposure to phthalates and bisphenols on cardiometabolic outcomes in pregnancy and childhood. Reprod. Toxicol. 2017, 68, 105–118. [Google Scholar] [CrossRef] [Green Version]
- Kawano, M.; Qin, X.Y.; Yoshida, M.; Fukuda, T.; Nansai, H.; Hayashi, Y.; Nakajima, T.; Sone, H. Peroxisome proliferator-activated receptor alpha mediates di-(2-ethylhexyl) phthalate transgenerational repression of ovarian Esr1 expression in female mice. Toxicol. Lett. 2014, 228, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.S.; Schaedlich, K.; Fiandanese, N.; Pocar, P.; Fischer, B. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environ. Health Perspect. 2012, 120, 1123–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovekamp-Swan, T.; Jetten, A.M.; Davis, B.J. Dual activation of PPARalpha and PPARgamma by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Mol. Cell Endocrinol. 2003, 201, 133–141. [Google Scholar] [CrossRef]
- Xu, C.; Chen, J.A.; Qiu, Z.; Zhao, Q.; Luo, J.; Yang, L.; Zeng, H.; Huang, Y.; Zhang, L.; Cao, J.; et al. Ovotoxicity and PPAR-mediated aromatase downregulation in female Sprague-Dawley rats following combined oral exposure to benzo [a]pyrene and di-(2-ethylhexyl) phthalate. Toxicol. Lett. 2010, 199, 323–332. [Google Scholar] [CrossRef]
- Ford, J.H. Reduced quality and accelerated follicle loss with female reproductive aging—Does decline in theca dehydroepiandrosterone (DHEA) underlie the problem? J. Biomed. Sci. 2013, 20, 93. [Google Scholar] [CrossRef] [Green Version]
- Eftekhari Moghadam, A.R.; Saki, G.; Hemadi, M.; Mazaheri, Z.; Khodadadi, A. Effect of dehydroepiandrosterone on meiotic spindle structure and oocyte quality in mice. Iran. J. Basic Med. Sci. 2018, 21, 1020–1025. [Google Scholar]
- Zhao, Y.; Tan, Y.S.; Strynar, M.J.; Perez, G.; Haslam, S.Z.; Yang, C. Perfluorooctanoic acid effects on ovaries mediate its inhibition of peripubertal mammary gland development in Balb/c and C57Bl/6 mice. Reprod. Toxicol. 2012, 33, 563–576. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.F.; Nosjean, O.; Cozzone, D.; D’Orazio, D.; Becchi, M.; Guichardant, M.; Ferry, G.; Boutin, J.A.; Lagarde, M.; Geloen, A. Covalent binding of 15-deoxy-delta12,14-prostaglandin J2 to PPARgamma. Biochem. Biophys. Res. Commun. 2005, 337, 521–525. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Wu, J. 15-Deoxy-(12,14)-Prostaglandin J2 (15d-PGJ2), an Endogenous Ligand of PPAR-γ: Function and Mechanism. PPAR Res. 2019, 2019, 7242030. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, M.; Capobianco, E.; Careaga, V.; Martinez, N.; Mazzucco, M.B.; Maier, M.; Jawerbaum, A. Peroxisome proliferator-activated receptor ligands regulate lipid content, metabolism, and composition in fetal lungs of diabetic rats. J. Endocrinol. 2014, 220, 345–359. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.M.; Cook, J.A.; Hake, P.W.; O’Connor, M.; Burroughs, T.J.; Zingarelli, B. 15-Deoxy-delta(12,14)-prostaglandin J(2) (15D-PGJ(2)), a peroxisome proliferator activated receptor gamma ligand, reduces tissue leukosequestration and mortality in endotoxic shock. Shock 2005, 24, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Horner, C.M. In vivo exposure of female rats to toxicants may affect oocyte quality. Reprod. Toxicol. 2003, 17, 273–281. [Google Scholar] [CrossRef]
- Makris, S.L.; Scott, C.S.; Fox, J.; Knudsen, T.B.; Hotchkiss, A.K.; Arzuaga, X.; Euling, S.Y.; Powers, C.M.; Jinot, J.; Hogan, K.A.; et al. A systematic evaluation of the potential effects of trichloroethylene exposure on cardiac development. Reprod. Toxicol. 2016, 65, 321–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, C.S.; Miranda-Alves, L.; La Merrill, M.A.; Silva, I.V.; Graceli, J.B. The tributyltin leads to obesogenic mammary gland abnormalities in adult female rats. Toxicol. Lett. 2019, 307, 59–71. [Google Scholar] [CrossRef]
- Crawford, K.A.; Clark, B.W.; Heiger-Bernays, W.J.; Karchner, S.I.; Hahn, M.E.; Nacci, D.E.; Schlezinger, J.J. Tributyltin disrupts fin development in Fundulus heteroclitus from both PCB-sensitive and resistant populations: Investigations of potential interactions between AHR and PPARgamma. Aquat. Toxicol. 2020, 218, 105334. [Google Scholar] [CrossRef]
- Hsueh, W.A.; Law, R. The central role of fat and effect of peroxisome proliferator-activated receptor-gamma on progression of insulin resistance and cardiovascular disease. Am. J. Cardiol. 2003, 92, 3J–9J. [Google Scholar] [CrossRef]
- Dennis, J.M.; Henley, W.E.; Weedon, M.N.; Lonergan, M.; Rodgers, L.R.; Jones, A.G.; Hamilton, W.T.; Sattar, N.; Janmohamed, S.; Holman, R.R.; et al. Sex and BMI Alter the Benefits and Risks of Sulfonylureas and Thiazolidinediones in Type 2 Diabetes: A Framework for Evaluating Stratification Using Routine Clinical and Individual Trial Data. Diabetes Care 2018, 41, 1844–1853. [Google Scholar] [CrossRef] [Green Version]
- Mohiyiddeen, L.; Watson, A.J.; Apostolopoulos, N.V.; Berry, R.; Alexandraki, K.I.; Jude, E.B. Effects of low-dose metformin and rosiglitazone on biochemical, clinical, metabolic and biophysical outcomes in polycystic ovary syndrome. J. Obstet. Gynaecol. 2013, 33, 165–170. [Google Scholar] [CrossRef]
- Vigerust, N.F.; Bohov, P.; Bjorndal, B.; Seifert, R.; Nygard, O.; Svardal, A.; Glintborg, D.; Berge, R.K.; Gaster, M. Free carnitine and acylcarnitines in obese patients with polycystic ovary syndrome and effects of pioglitazone treatment. Fertil. Steril. 2012, 98, 1620–1626 e1. [Google Scholar] [CrossRef]
- Minge, C.E.; Bennett, B.D.; Norman, R.J.; Robker, R.L. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reverses the adverse effects of diet-induced obesity on oocyte quality. Endocrinology 2008, 149, 2646–2656. [Google Scholar] [CrossRef] [Green Version]
- Rak-Mardyla, A.; Karpeta, A. Rosiglitazone stimulates peroxisome proliferator-activated receptor gamma expression and directly affects in vitro steroidogenesis in porcine ovarian follicles. Theriogenology 2014, 82, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kurzynska, A.; Bogacki, M.; Chojnowska, K.; Bogacka, I. Peroxisome proliferator activated receptor ligands affect progesterone and 17beta-estradiol secretion by porcine corpus luteum during early pregnancy. J. Physiol. Pharmacol. 2014, 65, 709–717. [Google Scholar]
- Toda, K.; Okada, T.; Miyaura, C.; Saibara, T. Fenofibrate, a ligand for PPARalpha, inhibits aromatase cytochrome P450 expression in the ovary of mouse. J. Lipid Res. 2003, 44, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Raheem, I.T.; Omran, G.A.; Katary, M.A. Irbesartan, an angiotensin II receptor antagonist, with selective PPAR-γ-modulating activity improves function and structure of chemotherapy-damaged ovaries in rats. Fundam. Clin. Pharmacol. 2015, 29, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Polkowski, K.; Mazurek, A.P. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol. Pharm. 2000, 57, 135–155. [Google Scholar] [PubMed]
- Lv, Z.; Fan, H.; Zhang, B.; Ning, C.; Xing, K.; Guo, Y. Dietary genistein supplementation in laying broiler breeder hens alters the development and metabolism of offspring embryos as revealed by hepatic transcriptome analysis. FASEB J. 2018, 32, 4214–4228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARgamma): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.J.; Hwang, S.J.; Yoon, J.A.; Jun, J.H.; Lim, H.J.; Yoon, T.K.; Song, H. Activation of peroxisome proliferators-activated receptor delta (PPARdelta) promotes blastocyst hatching in mice. Mol. Hum. Reprod. 2011, 17, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Heaney, A.P.; Fernando, M.; Melmed, S. PPAR-γ receptor ligands: Novel therapy for pituitary adenomas. J. Clin. Investig. 2003, 111, 1381–1388. [Google Scholar] [CrossRef]
- Chang, S.N.; Lee, J.M.; Oh, H.; Kim, U.; Ryu, B.; Park, J.H. Troglitazone inhibits the migration and invasion of PC-3 human prostate cancer cells by upregulating E-cadherin and glutathione peroxidase 3. Oncol. Lett. 2018, 16, 5482–5488. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Iyengar, S.; Roberts, R.L.; Shappell, S.B.; Peehl, D.M. Primary culture model of peroxisome proliferator-activated receptor gamma activity in prostate cancer cells. J. Cell Physiol. 2003, 196, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Stephen, R.L.; Gustafsson, M.C.; Jarvis, M.; Tatoud, R.; Marshall, B.R.; Knight, D.; Ehrenborg, E.; Harris, A.L.; Wolf, C.R.; Palmer, C.N. Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 2004, 64, 3162–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Zhai, Y.; Wang, J. The Role of PPAR and Its Cross-Talk with CAR and LXR in Obesity and Atherosclerosis. Int. J. Mol. Sci. 2018, 19, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahansouz, C.; Xu, H.; Hertzel, A.V.; Kizy, S.; Steen, K.A.; Foncea, R.; Serrot, F.J.; Kvalheim, N.; Luthra, G.; Ewing, K.; et al. Partitioning of adipose lipid metabolism by altered expression and function of PPAR isoforms after bariatric surgery. Int. J. Obes. 2018, 42, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xu, P.; Xie, X.; Li, J.; Zhang, J.; Wang, J.; Hong, F.; Li, J.; Zhang, Y.; Song, Y.; et al. DBZ (Danshensu Bingpian Zhi), a Novel Natural Compound Derivative, Attenuates Atherosclerosis in Apolipoprotein E-Deficient Mice. J. Am. Heart Assoc. 2017, 6, e006297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levak-Frank, S.; Radner, H.; Walsh, A.; Stollberger, R.; Knipping, G.; Hoefler, G.; Sattler, W.; Weinstock, P.H.; Breslow, J.L.; Zechner, R. Muscle-specific overexpression of lipoprotein lipase causes a severe myopathy characterized by proliferation of mitochondria and peroxisomes in transgenic mice. J. Clin. Investig. 1995, 96, 976–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoefler, G.; Noehammer, C.; Levak-Frank, S.; el-Shabrawi, Y.; Schauer, S.; Zechner, R.; Radner, H. Muscle-specific overexpression of human lipoprotein lipase in mice causes increased intracellular free fatty acids and induction of peroxisomal enzymes. Biochimie 1997, 79, 163–168. [Google Scholar] [CrossRef]
- Hodel, C. Myopathy and rhabdomyolysis with lipid-lowering drugs. Toxicol. Lett. 2002, 128, 159–168. [Google Scholar] [CrossRef]
- Motojima, K.; Seto, K. Fibrates and statins rapidly and synergistically induce pyruvate dehydrogenase kinase 4 mRNA in the liver and muscles of mice. Biol. Pharm. Bull. 2003, 26, 954–958. [Google Scholar] [CrossRef] [Green Version]
- Toffoli, B.; Gilardi, F.; Winkler, C.; Soderberg, M.; Kowalczuk, L.; Arsenijevic, Y.; Bamberg, K.; Bonny, O.; Desvergne, B. Nephropathy in Pparg-null mice highlights PPARgamma systemic activities in metabolism and in the immune system. PLoS ONE 2017, 12, e0171474. [Google Scholar] [CrossRef] [Green Version]
- Corrales, P.; Izquierdo-Lahuerta, A.; Medina-Gomez, G. Maintenance of Kidney Metabolic Homeostasis by PPAR Gamma. Int. J. Mol. Sci. 2018, 19, 2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Lee, S.; Chang, S.C.; Lee, J. Significant roles of neuroinflammation in Parkinson’s disease: Therapeutic targets for PD prevention. Arch. Pharm Res. 2019, 42, 416–425. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, M.; Antonosante, A.; Castelli, V.; Catanesi, M.; Moorthy, N.; Iannotta, D.; Cimini, A.; Benedetti, E. PPARs and Energy Metabolism Adaptation during Neurogenesis and Neuronal Maturation. Int. J. Mol. Sci. 2018, 19, 1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paintlia, A.S.; Paintlia, M.K.; Singh, A.K.; Singh, I. Modulation of Rho-Rock signaling pathway protects oligodendrocytes against cytokine toxicity via PPAR-alpha-dependent mechanism. Glia 2013, 61, 1500–1517. [Google Scholar] [CrossRef] [Green Version]
- Panlilio, L.V.; Justinova, Z.; Mascia, P.; Pistis, M.; Luchicchi, A.; Lecca, S.; Barnes, C.; Redhi, G.H.; Adair, J.; Heishman, S.J.; et al. Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: Preclinical findings. Neuropsychopharmacology 2012, 37, 1838–1847. [Google Scholar] [CrossRef] [Green Version]
- Porta, N.; Vallee, L.; Lecointe, C.; Bouchaert, E.; Staels, B.; Bordet, R.; Auvin, S. Fenofibrate, a peroxisome proliferator-activated receptor-alpha agonist, exerts anticonvulsive properties. Epilepsia 2009, 50, 943–948. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Y.; Wang, Y.; Li, D.; Liu, J.; Zhang, F. Age-Associated Dopaminergic Neuron Loss and Midbrain Glia Cell Phenotypic Polarization. Neuroscience 2019, 415, 89–96. [Google Scholar] [CrossRef]
- Loane, D.J.; Byrnes, K.R. Role of microglia in neurotrauma. Neurotherapeutics 2010, 7, 366–377. [Google Scholar] [CrossRef]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Le Menn, G.; Neels, J.G. Regulation of Immune Cell Function by PPARs and the Connection with Metabolic and Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 1575. [Google Scholar] [CrossRef] [Green Version]
- Dehmer, T.; Heneka, M.T.; Sastre, M.; Dichgans, J.; Schulz, J.B. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J. Neurochem. 2004, 88, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Breidert, T.; Callebert, J.; Heneka, M.T.; Landreth, G.; Launay, J.M.; Hirsch, E.C. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J. Neurochem. 2002, 82, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Schnegg, C.I.; Kooshki, M.; Hsu, F.C.; Sui, G.; Robbins, M.E. PPARdelta prevents radiation-induced proinflammatory responses in microglia via transrepression of NF-kappaB and inhibition of the PKCalpha/MEK1/2/ERK1/2/AP-1 pathway. Free Radic. Biol. Med. 2012, 52, 1734–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Choi, S.M.; Kim, B.C. Adiponectin Regulates the Polarization and Function of Microglia via PPAR-γ Signaling under Amyloid β Toxicity. Front. Cell Neurosci. 2017, 11, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Generic Name (Brand Name) | Type of PPAR Agonist | Molecular Weight and Molecular Formula | Structure | Company | Indications | Adverse Reaction or Toxicity |
|---|---|---|---|---|---|---|
| Rosiglitazone maleate (Avandia) | PPARγ agonist | 473.5 C22H23N3O7S |  | GlaxoSmithKline | Diabetes | Headache, cough, cold symptoms, and back pain |
| Pioglitazone hydrochloride (Actos) | PPARγ agonist | 392.898 C19H21ClN2O3S |  | Takeda/Lilly | Diabetes | Cold or flu-like symptoms, headache, gradual weight gain, muscle pain, back pain, tooth problems, and mouth pain |
| Lobeglitazone sulfate (Duvie) | Dual PPARα/γ agonist | 578.61 C24H26N4O9S2 |  | Chong Kun Dang | Diabetes | Edema and weight gain |
| Alogliptin benzoate/pioglitazone hydrochloride (Oseni) | Dipeptidyl peptidase IV inhibitor/PPARγ agonist | 461.519(C25H27N5O4)/392.898 (C19H21ClN2O3S) |  | Takeda | Diabetes | Upper respiratory tract infection, bone fracture, headache, nasopharyngitis, and pharyngitis |
| Glimepiride/pioglitazone hydrochloride (Duetact) | Sulfonylurea receptor modulator/PPARγ agonist | 490.62(C24H34N4O5S)/392.898 (C19H21ClN2O3S) |  | Takeda | Diabetes | Congestive heart failure, hypoglycemia, edema, fractures, and hemolytic anemia |
| Pioglitazone hydrochloride/metformin hydrochloride (Actoplus Met) | PPARγ agonist/ adenosine monophosphate-activated protein kinase (AMPK) activator | 392.898 (C19H21ClN2O3S)/165.6(C4H12ClN5) |  | Takeda | Diabetes | Headache, nausea, vomiting, stomach upset, diarrhea, weakness, sore throat, muscle pain, weight gain, tooth problems, a metallic taste in the mouth, and sneezing, runny nose, cough, or other signs of a cold |
| Rosiglitazone maleate/metformin hydrochloride (Avandamet) | PPARγ agonist; AMPK activator | 473.5(C22H23N3O7S)/165.6(C4H12ClN5) |  | GlaxoSmithKline | Diabetes | Lactic acidosis, cardiac failure, adverse cardiovascular events, edema, weight gain, hepatic effects, macular edema, fractures, hematologic effects, and ovulation |
| Glimepiride/rosiglitazone maleate (Avandaryl) | Sulfonylurea receptor modulator/PPARγ agonist | 490.62 (C24H34N4O5S)/473.5(C22H23N3O7S) |  | GlaxoSmithKline | Diabetes | Cardiac failure with rosiglitazone, major adverse cardiovascular events, hypoglycemia, edema, weight gain, hepatic effects, macular edema, fractures, hypersensitivity reactions, hematologic effects, hemolytic anemia, and increased risk of cardiovascular mortality for sulfonylurea drugs |
| Clofibrate (Atromid-S) | PPARα agonist | 242.699 C12H15ClO3 |  | Pfizer | Hyperlipidemia Hypertriglyceridemia Hypercholesterolemia | Common: diarrhea, nausea Rare: abnormal heart rhythm, acute inflammation of the pancreas, anemia, angina, gallstones, kidney failure, and low levels of white blood cells |
| Fenofibrate (Antara) | PPARα agonist | 360.834 C20H21ClO4 |  | Abbvie | Hypercholesterolemia Hypertriglyceridemia | Common: abnormal liver tests including aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and headache Rare: high blood pressure, dizziness, itching, nausea, upset stomach, constipation, diarrhea, urinary tract infections, muscle pain, kidney problems, and respiratory tract infections |
| Choline fenofibrate (Fenofibric Acid) | PPARα agonist | 421.918 C22H28ClNO5 |  | Abbvie | Hyperlipidemia | Diarrhea, dyspepsia, nasopharyngitis, sinusitis, upper respiratory tract infection, arthralgia, myalgia, pain in extremities, dizziness |
| Bezafibrate (Bezalip) | PPARα agonist | 361.822 C19H20ClNO4 |  | Roche Diagnostics | Hypertriglyceridemia hypercholesterolemia mixed hyperlipidemia | Stomach upset, stomach pain, gas, or nausea may occur in the first several days; itchy skin, redness, headache, and dizziness |
| Gemfibrozil (Lopid) | PPARα agonist | 250.338 C15H22O3 |  | Pfizer | Hyperlipidemia Ischemic heart disorder | Stomach upset, stomach/abdominal pain, nausea, vomiting, diarrhea, constipation, rash, dizziness, headache, changes in the way things taste, muscle pain |
| Ciprofibrate (Lipanor) | PPARα agonist | 289.152 C13H14Cl2O3 |  | Sanofi-Aventis | Hyperlipidemia | Hair loss, balding, headache, balance problems, feeling dizzy, drowsiness or fatigue, feeling sick (nausea) or being sick (vomiting), diarrhea, indigestion or stomach pains, muscle pains |
| Pemafibrate (Parmodia) | PPARα agonist | 490.556 C28H30N2O6 |  | Kowa | Dyslipidemia | Cholelithiasis (upper abdominal pain, fever) and diabetes mellitus (dry mouth, excess intake of fluid, excessive urination, fatigue) |
| Pravastatin sodium/fenofibrate (Pravafenix) | 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) inhibitor/PPARα agonist | 446.5(C23H35NaO7)/360.83(C20H21ClO4) |  | Laboratoires SMB | Mixed hyperlipidemia Coronary heart disease | Abdominal distension (bloating), abdominal pain (stomach ache), constipation, diarrhea, dry mouth, dyspepsia (heartburn), eructation (belching), flatulence (gas), nausea (feeling sick), abdominal discomfort, vomiting, and raised blood levels of liver enzymes |
| Fenofibrate/simvastatin (Cholib) | PPARα agonist/ HMGCR inhibitor | 360.834 (C20H21ClO4)/418.57(C25H38O5) |  | Mylan | Mixed hyperlipidemia | Raised blood creatinine levels, upper-respiratory-tract infection (colds), increased blood platelet counts, gastroenteritis (diarrhea and vomiting) and increased levels of alanine aminotransferase |
| Saroglitazar (Lipaglyn) | PPARα/γ agonist | 439.57 C25H29NO4S |  | Zydus Cadila | Diabetic dyslipidemia | Asthenia, gastritis, chest discomfort, peripheral edema, dizziness, and tremors |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Y.; Zhang, Y.; Zhu, S.; Luo, Y.; Xu, P.; Huang, Z. PPAR-Mediated Toxicology and Applied Pharmacology. Cells 2020, 9, 352. https://doi.org/10.3390/cells9020352
Xi Y, Zhang Y, Zhu S, Luo Y, Xu P, Huang Z. PPAR-Mediated Toxicology and Applied Pharmacology. Cells. 2020; 9(2):352. https://doi.org/10.3390/cells9020352
Chicago/Turabian StyleXi, Yue, Yunhui Zhang, Sirui Zhu, Yuping Luo, Pengfei Xu, and Zhiying Huang. 2020. "PPAR-Mediated Toxicology and Applied Pharmacology" Cells 9, no. 2: 352. https://doi.org/10.3390/cells9020352