Umbilical Cord Mesenchymal Stromal/Stem Cells and Their Interplay with Th-17 Cell Response Pathway
Abstract
:1. Introduction
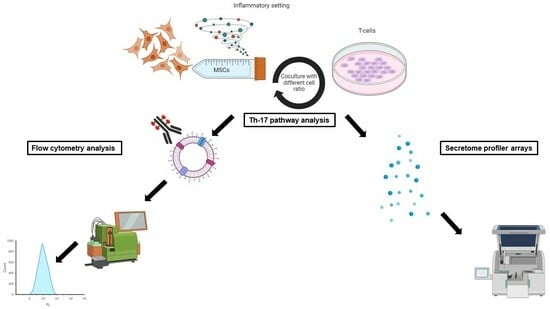
2. Materials and Methods
2.1. Isolation and Culture of UC-MSCs
2.2. Phenotype of UC-MSCs
2.3. In Vitro Trilineage Potential of UC-MSCs
2.3.1. Osteogenic Differentiation
2.3.2. Adipogenic Differentiation
2.3.3. Chondrogenic Differentiation
2.4. Inflammatory Preconditioning of UC-MSCs
2.5. Immune Cells
2.6. Coculture of Immune Cells with UC-MSCs
2.7. Morphology of Cocultured Cell Populations
2.8. IL-17A Expression
2.9. RORγt Expression
2.10. IL-23 Receptor Expression
2.11. Cytokine Secretion Profile
2.12. Flow Cytometry
2.13. Construction and Analysis of the Protein–Protein Interaction Network
2.14. Statistical Analysis
3. Results
3.1. Characterization of UC-MSCs
3.1.1. The Phenotype of UC-MSCs
3.1.2. In Vitro Trilineage Potential of UC-MSCs
3.1.3. Morphology of Cocultured Cell Populations
3.2. Expression of IL-17A in T Cells during Coculture with UC-MSCs
3.3. Expression of RORγt in T Cells during Coculture with UC-MSCs
3.4. Expression of the IL-23 Receptor in T Cells during Coculture with UC-MSCs
3.5. Th-17-Associated Cytokine Profile
4. Discussion
5. Conclusions
Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velarde, F.; Castañeda, V.; Morales, E.; Ortega, M.; Ocaña, E.; Álvarez-Barreto, J.; Grunauer, M.; Eguiguren, L.; Caicedo, A. Use of Human Umbilical Cord and Its Byproducts in Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Guan, H.; Zhou, F. Biological Characteristics of Umbilical Cord Mesenchymal Stem Cells and Its Therapeutic Potential for Hematological Disorders. Front. Cell Dev. Biol. 2021, 9, 570179. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound healing-aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Phinney, D.G.; Hwa Lee, R.; Boregowda, S.V. Revisiting the Mesenchymal “Stem vs. Stromal” Cell Dichotomy and Its Implications for Development of Improved Potency Metrics. Stem Cells 2023, 41, 444–452. [Google Scholar] [CrossRef]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Planat-Benard, V.; Varin, A.; Casteilla, L. MSCs and Inflammatory Cells Crosstalk in Regenerative Medicine: Concerted Actions for Optimized Resolution Driven by Energy Metabolism. Front. Immunol. 2021, 12, 626755. [Google Scholar] [CrossRef]
- Brockmann, L.; Giannou, A.D.; Gagliani, N.; Huber, S. Regulation of T(H)17 Cells and Associated Cytokines in Wound Healing, Tissue Regeneration, and Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1033. [Google Scholar] [CrossRef]
- Martinez, G.J.; Nurieva, R.I.; Yang, X.O.; Dong, C. Regulation and function of proinflammatory TH17 cells. Ann. N. Y. Acad. Sci. 2008, 1143, 188–211. [Google Scholar] [CrossRef] [PubMed]
- Capone, A.; Volpe, E. Transcriptional Regulators of T Helper 17 Cell Differentiation in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Crompot, E.; van Grunsven, L.A.; Dollé, L.; Lagneaux, L. Aldehyde dehydrogenase activity of Wharton jelly mesenchymal stromal cells: Isolation and characterization. Cytotechnology 2019, 71, 427–441. [Google Scholar] [CrossRef]
- Buyl, K.; Vanhaecke, T.; Desmae, T.; Lagneaux, L.; Rogiers, V.; Najar, M.; De Kock, J. Evaluation of a new standardized enzymatic isolation protocol for human umbilical cord-derived stem cells. Toxicol. In Vitro 2015, 29, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Merimi, M.; Faour, W.H.; Lombard, C.A.; Moussa Agha, D.; Ouhaddi, Y.; Sokal, E.M.; Lagneaux, L.; Fahmi, H. In Vitro Cellular and Molecular Interplay between Human Foreskin-Derived Mesenchymal Stromal/Stem Cells and the Th17 Cell Pathway. Pharmaceutics 2021, 13, 1736. [Google Scholar] [CrossRef] [PubMed]
- STRING. Welcome to STRING. Available online: http://string-db.org;version11.0 (accessed on 14 August 2023).
- Bouhtit, F.; Najar, M.; Rahmani, S.; Melki, R.; Najimi, M.; Sadki, K.; Boukhatem, N.; Twizere, J.C.; Meuleman, N.; Lewalle, P.; et al. Bioscreening and pre-clinical evaluation of the impact of bioactive molecules from Ptychotis verticillata on the multilineage potential of mesenchymal stromal cells towards immune- and inflammation-mediated diseases. Inflamm. Res. 2022, 71, 887–898. [Google Scholar] [CrossRef]
- Mishra, S.; Sevak, J.K.; Das, A.; Arimbasseri, G.A.; Bhatnagar, S.; Gopinath, S.D. Umbilical cord tissue is a robust source for mesenchymal stem cells with enhanced myogenic differentiation potential compared to cord blood. Sci. Rep. 2020, 10, 18978. [Google Scholar] [CrossRef] [PubMed]
- Ayavoo, T.; Murugesan, K.; Gnanasekaran, A. Roles and mechanisms of stem cell in wound healing. Stem Cell Investig. 2021, 8, 4. [Google Scholar] [CrossRef]
- Merimi, M.; El-Majzoub, R.; Lagneaux, L.; Moussa Agha, D.; Bouhtit, F.; Meuleman, N.; Fahmi, H.; Lewalle, P.; Fayyad-Kazan, M.; Najar, M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front. Cell Dev. Biol. 2021, 9, 661532. [Google Scholar] [CrossRef]
- Mebarki, M.; Iglicki, N.; Marigny, C.; Abadie, C.; Nicolet, C.; Churlaud, G.; Maheux, C.; Boucher, H.; Monsel, A.; Menasché, P.; et al. Development of a human umbilical cord-derived mesenchymal stromal cell-based advanced therapy medicinal product to treat immune and/or inflammatory diseases. Stem Cell Res. Ther. 2021, 12, 571. [Google Scholar] [CrossRef]
- Broekman, W.; Amatngalim, G.D.; de Mooij-Eijk, Y.; Oostendorp, J.; Roelofs, H.; Taube, C.; Stolk, J.; Hiemstra, P.S. TNF-α and IL-1β-activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor. Respir. Res. 2016, 17, 3. [Google Scholar] [CrossRef]
- Cerboni, S.; Gehrmann, U.; Preite, S.; Mitra, S. Cytokine-regulated Th17 plasticity in human health and diseases. Immunology 2021, 163, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Huang, Y.; Bao, T.; Liu, C.; Liu, X.; Chen, X. The role of Th17 cells/IL-17A in AD, PD, ALS and the strategic therapy targeting on IL-17A. J. Neuroinflammation 2022, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.A.B.; Dagamajalu, S.; Gouda, M.M.; Suchitha, G.P.; Chanderasekaran, J.; Raju, R.; Prasad, T.S.K.; Bhandary, Y.P. A comprehensive network map of IL-17A signaling pathway. J. Cell Commun. Signal. 2023, 17, 209–215. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Boldrini, V.; dos Santos Farias, A.; Degasperi, G.R. Deciphering targets of Th17 cells fate: From metabolism to nuclear receptors. Scand. J. Immunol. 2019, 90, e12793. [Google Scholar] [CrossRef]
- Khanam, A.; Trehanpati, N.; Sarin, S.K. Increased interleukin-23 receptor (IL-23R) expression is associated with disease severity in acute-on-chronic liver failure. Liver Int. 2019, 39, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Lombard, C.A.; Fayyad-Kazan, H.; Faour, W.H.; Merimi, M.; Sokal, E.M.; Lagneaux, L.; Fahmi, H. Th17 immune response to adipose tissue-derived mesenchymal stromal cells. J. Cell Physiol. 2019, 234, 21145–21152. [Google Scholar] [CrossRef]
- Najar, M.; Fayyad-Kazan, H.; Faour, W.H.; Merimi, M.; Sokal, E.M.; Lombard, C.A.; Fahmi, H. Immunological modulation following bone marrow-derived mesenchymal stromal cells and Th17 lymphocyte co-cultures. Inflamm. Res. 2019, 68, 203–213. [Google Scholar] [CrossRef]
- Chong, W.P.; Mattapallil, M.J.; Raychaudhuri, K.; Bing, S.J.; Wu, S.; Zhong, Y.; Wang, W.; Chen, Z.; Silver, P.B.; Jittayasothorn, Y.; et al. The Cytokine IL-17A Limits Th17 Pathogenicity via a Negative Feedback Loop Driven by Autocrine Induction of IL-24. Immunity 2020, 53, 384–397.e385. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Zhu, Y.; Liu, X.; Gu, Y.; Dai, X.; Li, B. Transcriptional and posttranslational regulation of Th17/Treg balance in health and disease. Eur. J. Immunol. 2021, 51, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Pokrovskii, M.; Kroehling, L.; Kim, B.R.; Kim, S.Y.; Wu, L.; Lee, J.Y.; Littman, D.R. Transcription factor RORα enforces stability of the Th17 cell effector program by binding to a Rorc cis-regulatory element. Immunity 2022, 55, 2027–2043.e2029. [Google Scholar] [CrossRef]
- Stadhouders, R.; Lubberts, E.; Hendriks, R.W. A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J. Autoimmun. 2018, 87, 1–15. [Google Scholar] [CrossRef]
- Qu, N.; Xu, M.; Mizoguchi, I.; Furusawa, J.; Kaneko, K.; Watanabe, K.; Mizuguchi, J.; Itoh, M.; Kawakami, Y.; Yoshimoto, T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin. Dev. Immunol. 2013, 2013, 968549. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Huang, M.; Yao, Y.M. Biology of Interleukin-17 and Its Pathophysiological Significance in Sepsis. Front. Immunol. 2020, 11, 1558. [Google Scholar] [CrossRef]
- Cole, S.; Murray, J.; Simpson, C.; Okoye, R.; Tyson, K.; Griffiths, M.; Baeten, D.; Shaw, S.; Maroof, A. Interleukin (IL)-12 and IL-18 Synergize to Promote MAIT Cell IL-17A and IL-17F Production Independently of IL-23 Signaling. Front. Immunol. 2020, 11, 585134. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Pușcaș, A.D.; Morar, I.I.; Vesa, Ș.C.; Cătană, A.; Pușcaș, C.; Ilieș, R.F.; Orasan, R.-I. Association between IL-17F, IL-17RA Gene Polymorphisms and Response to Biological Drugs in Psoriasis and Beyond. Genes 2023, 14, 1123. [Google Scholar] [CrossRef]
- Schminke, B.; Trautmann, S.; Mai, B.; Miosge, N.; Blaschke, S. Interleukin 17 inhibits progenitor cells in rheumatoid arthritis cartilage. Eur. J. Immunol. 2016, 46, 440–445. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Intosalmi, J.; Ahlfors, H.; Rautio, S.; Mannerstöm, H.; Chen, Z.J.; Lahesmaa, R.; Stockinger, B.; Lähdesmäki, H. Analyzing Th17 cell differentiation dynamics using a novel integrative modeling framework for time-course RNA sequencing data. BMC Syst. Biol. 2015, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- McGee, H.M.; Schmidt, B.A.; Booth, C.J.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Stevens, S.; Flavell, R.A.; Horsley, V. IL-22 promotes fibroblast-mediated wound repair in the skin. J. Investig. Dermatol. 2013, 133, 1321–1329. [Google Scholar] [CrossRef]
- Lindahl, H.; Olsson, T. Interleukin-22 Influences the Th1/Th17 Axis. Front. Immunol. 2021, 12, 618110. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef]
- Schinocca, C.; Rizzo, C.; Fasano, S.; Grasso, G.; La Barbera, L.; Ciccia, F.; Guggino, G. Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Front. Immunol. 2021, 12, 637829. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Heise, D.; Derrac Soria, A.; Hansen, S.; Dambietz, C.; Akbarzadeh, M.; Berg, A.F.; Waetzig, G.H.; Jones, S.A.; Dvorsky, R.; Ahmadian, M.R.; et al. Selective inhibition of IL-6 trans-signaling by a miniaturized, optimized chimeric soluble gp130 inhibits TH17 cell expansion. Sci. Signal. 2021, 14, eabc3480. [Google Scholar] [CrossRef]
- Chang, H.; Zhao, F.; Xie, X.; Liao, Y.; Song, Y.; Liu, C.; Wu, Y.; Wang, Y.; Liu, D.; Wang, Y.; et al. PPARα suppresses Th17 cell differentiation through IL-6/STAT3/RORγt pathway in experimental autoimmune myocarditis. Exp. Cell Res. 2019, 375, 22–30. [Google Scholar] [CrossRef]
- Harbour, S.N.; DiToro, D.F.; Witte, S.J.; Zindl, C.L.; Gao, M.; Schoeb, T.R.; Jones, G.W.; Jones, S.A.; Hatton, R.D.; Weaver, C.T. TH17 cells require ongoing classic IL-6 receptor signaling to retain transcriptional and functional identity. Sci. Immunol. 2020, 5, eaaw2262. [Google Scholar] [CrossRef]
- Saini, C.; Srivastava, R.K.; Tarique, M.; Kurra, S.; Khanna, N.; Ramesh, V.; Sharma, A. Elevated IL-6R on CD4(+) T cells promotes IL-6 driven Th17 cell responses in patients with T1R leprosy reactions. Sci. Rep. 2020, 10, 15143. [Google Scholar] [CrossRef] [PubMed]
- Jawale, D.; Khandibharad, S.; Singh, S. Decoding systems immunological model of sphingolipids with IL-6/IL-17/IL-23 axes in L. major infection. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2023, 1868, 159261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gang, X.; Yang, S.; Cui, M.; Sun, L.; Li, Z.; Wang, G. The Alterations in and the Role of the Th17/Treg Balance in Metabolic Diseases. Front. Immunol. 2021, 12, 678355. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Chang, S.H.; Martinez, G.J.; Yang, X.O.; Nurieva, R.; Kang, H.S.; Ma, L.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 2009, 30, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.M.; Duarte, S.; Ribeiro, A.C.; Mascarenhas, P.; Noronha, S.; Alves, R.C. Association between IL-1A, IL-1B and IL-1RN Polymorphisms and Peri-Implantitis: A Systematic Review and Meta-Analysis. Appl. Sci. 2022, 12, 6958. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Huang, C.-H.; Wang, W.-H.; Tenhunen, J.; Hung, L.-C.; Lin, C.-C.; Chen, Y.-C.; Chen, Y.-H.; Liao, W.-T. Mono-(2-ethylhexyl) phthalate Promotes Dengue Virus Infection by Decreasing IL-23-Mediated Antiviral Responses. Front. Immunol. 2021, 12, 599345. [Google Scholar] [CrossRef]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Dermatol. Surg. 2016, 21, 14–19. [Google Scholar] [CrossRef]
- Nambi, G. Does low level laser therapy has effects on inflammatory biomarkers IL-1β, IL-6, TNF-α, and MMP-13 in osteoarthritis of rat models—A systemic review and meta-analysis. Lasers Med. Sci. 2021, 36, 475–484. [Google Scholar] [CrossRef]
- Schmitt, H.; Billmeier, U.; Dieterich, W.; Rath, T.; Sonnewald, S.; Reid, S.; Hirschmann, S.; Hildner, K.; Waldner, M.J.; Mudter, J.; et al. Expansion of IL-23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti-TNF therapy in Crohn’s disease. Gut 2019, 68, 814–828. [Google Scholar] [CrossRef]
- Erol, A.; Sel, F.A.; Süleymanoğlu, M.; Demirayak, G.; Kuruca, D.S.; Savran Oğuz, F. Evaluation of the relationship between mesenchymal stem cells and immune system in vitro conditions. Mol. Biol. Rep. 2023, 50, 4347–4356. [Google Scholar] [CrossRef]
- Bridgewood, C.; Newton, D.; Bragazzi, N.; Wittmann, M.; McGonagle, D. Unexpected connections of the IL-23/IL-17 and IL-4/IL-13 cytokine axes in inflammatory arthritis and enthesitis. Semin. Immunol. 2021, 58, 101520. [Google Scholar] [CrossRef] [PubMed]
- Faust, H.J.; Zhang, H.; Han, J.; Wolf, M.T.; Jeon, O.H.; Sadtler, K.; Peña, A.N.; Chung, L.; Maestas, D.R., Jr.; Tam, A.J.; et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J. Clin. Investig. 2020, 130, 5493–5507. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, R.; Sun, Y. Increased IFN-γ-producing Th17/Th1 cells and their association with lung function and current smoking status in patients with chronic obstructive pulmonary disease. BMC Pulm. Med. 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Boniface, K.; Blumenschein, W.M.; Brovont-Porth, K.; McGeachy, M.J.; Basham, B.; Desai, B.; Pierce, R.; McClanahan, T.K.; Sadekova, S.; de Waal Malefyt, R. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J. Immunol. 2010, 185, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.; Yu, D.; Cao, Z.; Zhao, X. Butyrate inhibit collagen-induced arthritis via Treg/IL-10/Th17 axis. Int. Immunopharmacol. 2019, 68, 226–233. [Google Scholar] [CrossRef]
- Yuan, Q.; Peng, N.; Xiao, F.; Shi, X.; Zhu, B.; Rui, K.; Tian, J.; Lu, L. New insights into the function of Interleukin-25 in disease pathogenesis. Biomark. Res. 2023, 11, 36. [Google Scholar] [CrossRef]
- Di Salvo, E.; Ventura-Spagnolo, E.; Casciaro, M.; Navarra, M.; Gangemi, S. IL-33/IL-31 Axis: A Potential Inflammatory Pathway. Mediators Inflamm. 2018, 2018, 3858032. [Google Scholar] [CrossRef]
- Korn, T.; Hiltensperger, M. Role of IL-6 in the commitment of T cell subsets. Cytokine 2021, 146, 155654. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef]










| Human Primary Antibody | Species | Dilution | Source | Isotype Control |
|---|---|---|---|---|
| Anti-CD73-APC | Mouse | 1/20 | BD Biosciences (San Jose, CA, USA) | APC mouse IgG1 |
| Anti-CD90-PE | Mouse | 1/20 | R&D Systems (Minneapolis, MN, USA) | PE mouse IgG2A |
| Anti-105-FITC | Mouse | 1/20 | BioLegend (San Diego, CA, USA) | FITC mouse IgG1 |
| Anti-CD34-PC5 | Mouse | 1/20 | BD Biosciences | PC5 mouse IgG1 |
| Anti-CD14-PE | Mouse | 1/20 | BD Biosciences | PE mouse IgG2A |
| Anti-CD19-PE | Mouse | 1/20 | BD Biosciences | PE mouse IgG1 |
| Anti-CD45-PC7 | Mouse | 1/20 | BD Biosciences | PC7 mouse IgG2A |
| Anti-HLA-DR-PerCP | Mouse | 1/20 | BD Biosciences | PerCP mouse IgG2A |
| Network Stats | ||||
|---|---|---|---|---|
| Number of nodes: 21 | Expected number of edges: 53 | |||
| Number of edges: 174 | PPI enrichment p-value: <1.0 × 10−16 | |||
| Average node degree: 16.6 | Your network has significantly more interactions | |||
| Avg. local clustering coefficient: 0.898 | than expected | |||
| Functional enrichments in your network | ||||
 | Biological Process (Gene Ontology) | |||
| GO-term | Description | Count in network | Strength | False-discovery rate |
| GO-1900100 | Positive regulation of plasma cell differentiation | 2 of 3 | 2.8 | 0.00098 |
| GO-0060559 | Positive regulation of calcidiol 1-monooxygenase activity | 2 of 3 | 2.8 | 0.00098 |
| GO-0001660 | Fever generation | 2 of 3 | 2.8 | 0.00098 |
| GO-2000340 | Positive regulation of chemokine (C-X-C motif) ligand 1 production | 2 of 5 | 2.75 | 1.00 × 10−5 |
| GO-0032747 | Positive regulation of interleukin-23 production | 2 of 7 | 2.6 | 1.89 × 10−5 |
 | Molecular Function (Gene Ontology) | |||
| GO-term | Description | Count in network | Strength | False-discovery rate |
| GO-0004920 | Positive regulation of plasma cell differentiation | 2 of 2 | 2.97 | 0.0020 |
| GO-0030368 | Positive regulation of calcidiol 1-monooxygenase activity | 2 of 8 | 2.55 | 7.02 × 10−5 |
| GO-0005149 | Fever generation | 2 of 17 | 2.04 | 0.0474 |
| GO-2004896 | Positive regulation of chemokine (C-X-C motif) ligand 1 production | 2 of 96 | 1.94 | 1.67 × 10−12 |
| GO-0070851 | Positive regulation of interleukin-23 production | 2 of 135 | 1.69 | 5.07 × 10−8 |
 | Cellular Component (Gene Ontology) | |||
| GO-term | Description | Count in network | Strength | False-discovery rate |
| GO-0098802 | Plasma membrane signaling receptor complex | 4 of 194 | 2.29 | 0.0280 |
| GO-0005887 | Integral component of plasma membrane | 9 of 1706 | 0.69 | 0.0280 |
| GO-0005615 | Extracellular space | 12 of 3247 | 0.54 | 0.0280 |
| GO-0005576 | Extracellular region | 14 of 4175 | 0.5 | 0.0192 |
| Network Stats | ||||
| Number of nodes: 21 | Expected number of edges: 47 | |||
| Number of edges: 177 | PPI enrichment p-value: <1.0 × 10−16 | |||
| Average node degree: 16.9 | Your network has significantly more interactions | |||
| Avg. local clustering coefficient: 0.903 | than expected | |||
| Functional enrichments in your network | ||||
 | Biological Process (Gene Ontology) | |||
| GO-term | Description | Count in network | Strength | False-discovery rate |
| GO-2000330 | Positive regulation of T-helper 17 cell lineage commitment | 4 of 4 | 2.97 | 1.08 × 10−8 |
| GO-0051142 | Positive regulation of NK T cell proliferation | 2 of 10 | 2.82 | 1.39 × 10−14 |
| GO-2000635 | Negative regulation of primary miRNA processing | 2 of 3 | 2.8 | 0.00068 |
| GO-1900100 | Positive regulation of plasma cell differentiation | 2 of 3 | 2.8 | 0.00068 |
| GO-0038155 | Interleukin-23-mediated signaling pathway | 2 of 3 | 2.8 | 0.00068 |
 | Molecular Function (Gene Ontology) | |||
| GO-term | Description | Count in network | Strength | False-discovery rate |
| GO-0004920 | Interleukin-10 receptor activity | 2 of 2 | 2.97 | 0.0021 |
| GO-0005143 | Interleukin-12 receptor binding | 2 of 3 | 2.8 | 0.0033 |
| GO-0004904 | Interferon receptor activity | 2 of 5 | 2.57 | 0.0066 |
| GO-0031702 | Type 1 angiotensin receptor binding | 2 of 6 | 2.5 | 0.0083 |
| GO-0005131 | Growth hormone receptor binding | 2 of 11 | 2.23 | 0.0206 |
 | Cellular Component (Gene Ontology) | |||
| GO-term | Description | Count in network | Strength | False-discovery rate |
| GO-0070743 | Plasma membrane signaling receptor complex | 2 of 2 | 2.97 | 0.0015 |
| GO-0072536 | Integral component of plasma membrane | 6 of 7 | 2.91 | 2.32 × 10−12 |
| GO-0042022 | Extracellular space | 5 of 7 | 2.83 | 4.41 × 10−10 |
| GO-0098802 | Extracellular region | 9 of 194 | 1.64 | 2.94 × 10−10 |
| GO-0009897 | External side of plasma membrane | 5 of 388 | 1.08 | 0.0098 |
| Network Stats | ||||
| Number of nodes: 21 | Expected number of edges: 36 | |||
| Number of edges: 106 | PPI enrichment p-value: < 1.0 × 10−16 | |||
| Average node degree: 10.1 | Your network has significantly more interactions | |||
| Avg: local clustering coefficient: 0.713 | than expected | |||
| Functional enrichments in your network | ||||
 | Biological Process (Gene Ontology) | |||
| GO-term | Description | Count in network | Strength | False-discovery rate |
| GO-1904017 | Cellular response to Thytoglobulin triiodothyronine | 2 of 3 | 2.8 | 0.0014 |
| GO-0018076 | N-terminal peptidyl-lysine acetylation | 2 of 3 | 2.8 | 0.0014 |
| GO-2000340 | Positive regulation of chemokine (C-X-C motif) ligand 1 production | 2 of 5 | 2.57 | 0.0024 |
| GO-1904179 | Positive regulation of adipose tissue development | 2 of 8 | 2.55 | 3.83 × 10−5 |
| GO-0035357 | Peroxisome proliferator activated receptor signaling pathway | 2 of 9 | 2.5 | 4.82 × 10−5 |
 | Molecular Function (Gene Ontology) | |||
| GO-term | Description | Count in network | Strength | False-discovery rate |
| GO-0017162 | Aryl hydrocarbon receptor binding | 3 of 9 | 2.5 | 4.88 × 10−5 |
| GO-0008142 | Oxysterol binding | 2 of 7 | 2.43 | 0.0042 |
| GO-0030368 | interleukin-17 receptor activity | 2 of 8 | 2.37 | 0.0052 |
| GO-0046965 | Nuclear retinoid X receptor binding | 3 of 14 | 2.3 | 0.00014 |
| GO-0097677 | STAT family protein binding | 1 | 2.23 | 0.0088 |
 | Cellular Component (Gene Ontology) | |||
| GO-term | Description | Count in network | Strength | False-discovery rate |
| GO-0017053 | Transcription repressor complex | 3 of 77 | 1.56 | 0.0111 |
| GO-0000118 | Histone deacetylase comples | 3 of 80 | 1.55 | 0.0116 |
| GO-0090575 | RNA polymerase II transcription regulator complex | 6 of 256 | 1.34 | 8.13 × 10−5 |
| GO-0005667 | Transcription regulator complex | 11 of 517 | 1.3 | 2.59 × 10−9 |
| GO-0000785 | Chromatin | 13 of 1285 | 0.98 | 5.20 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najar, M.; Rahmani, S.; Faour, W.H.; Alsabri, S.G.; Lombard, C.A.; Fayyad-Kazan, H.; Sokal, E.M.; Merimi, M.; Fahmi, H. Umbilical Cord Mesenchymal Stromal/Stem Cells and Their Interplay with Th-17 Cell Response Pathway. Cells 2024, 13, 169. https://doi.org/10.3390/cells13020169
Najar M, Rahmani S, Faour WH, Alsabri SG, Lombard CA, Fayyad-Kazan H, Sokal EM, Merimi M, Fahmi H. Umbilical Cord Mesenchymal Stromal/Stem Cells and Their Interplay with Th-17 Cell Response Pathway. Cells. 2024; 13(2):169. https://doi.org/10.3390/cells13020169
Chicago/Turabian StyleNajar, Mehdi, Saida Rahmani, Wissam H. Faour, Sami G. Alsabri, Catherine A. Lombard, Hussein Fayyad-Kazan, Etienne M. Sokal, Makram Merimi, and Hassan Fahmi. 2024. "Umbilical Cord Mesenchymal Stromal/Stem Cells and Their Interplay with Th-17 Cell Response Pathway" Cells 13, no. 2: 169. https://doi.org/10.3390/cells13020169