Effects of Acute and Chronic Gabapentin Treatment on Cardiovascular Function of Rats
Abstract
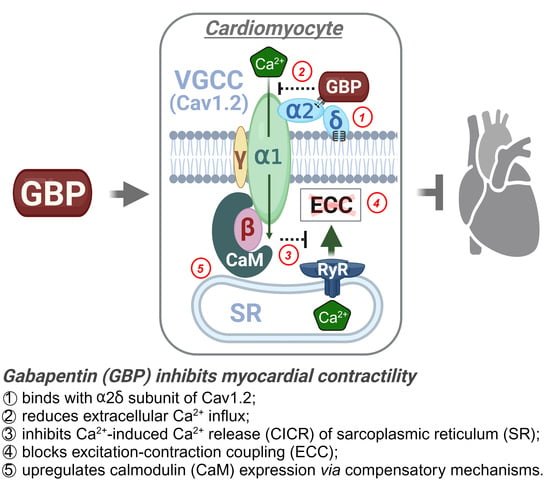
:1. Introduction
2. Materials and Methods
2.1. Arterial Blood Pressure (AP), Heart Rate (HR), and Left Ventricular (LV) Hemodynamics Measurements
2.2. Plasma Norepinephrine Assay
2.3. Proteomics Analysis
2.4. Bioinformatics Analysis
2.5. Western Blotting Analysis
2.6. Statistical Analyses
3. Results
3.1. Effect of Acute Bolus Intravenous Injection of GBP on Cardiovascular Function
3.2. Effect of Chronic i.p. Treatment of GBP on Cardiovascular Function
3.3. Plasma NE Concentration
3.4. Proteomics and Bioinformatics Analyses
3.5. Western Blotting Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baillie, J.K.; Power, I. The mechanism of action of gabapentin in neuropathic pain. Curr. Opin. Investig. Drugs 2006, 7, 33–39. [Google Scholar] [PubMed]
- Ziganshina, L.E.; Abakumova, T.; Hoyle, C.H. Gabapentin monotherapy for epilepsy: A review. Int. J. Risk Saf. Med. 2023, 34, 243–286. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.V.; Havens, J.R.; Walsh, S.L. Gabapentin misuse, abuse and diversion: A systematic review. Addiction 2016, 111, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Quintero, G.C. Review about gabapentin misuse, interactions, contraindications and side effects. J. Exp. Pharmacol. 2017, 9, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Calandre, E.P.; Rico-Villademoros, F.; Slim, M. Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: A review of their clinical pharmacology and therapeutic use. Expert Rev. Neurother. 2016, 16, 1263–1277. [Google Scholar] [CrossRef]
- Isom, L.L.; De Jongh, K.S.; Catterall, W.A. Auxiliary subunits of voltage-gated ion channels. Neuron 1994, 12, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef]
- Pan, Y.; Davis, P.B.; Kaebler, D.C.; Blankfield, R.P.; Xu, R. Cardiovascular risk of gabapentin and pregabalin in patients with diabetic neuropathy. Cardiovasc. Diabetol. 2022, 21, 170. [Google Scholar] [CrossRef]
- Park, S.H.; Hunter, K.; Berry, H.; Martins, Y.C. Atrial fibrillation induced by gabapentin: A case report. J. Med. Case Rep. 2023, 17, 236. [Google Scholar] [CrossRef]
- Al-bast, B.; Deshpande, R.; Hu, Y.Y.; Siddique, M. Gabapentin induced heart failure. J. Am. Coll. Cardiol. 2022, 79, 2231. [Google Scholar] [CrossRef]
- Barold, S.; Barold, D.; Hon, R. Gabapentin-Induced Congestive Heart Failure. Case Rep. Clin. Cardiol. J. 2023, 3, 130. [Google Scholar]
- Tellor, K.B.; Ngo-Lam, R.; Badran, D.; Armbruster, A.L.; Schwarze, M.W. A rare case of a gabapentin-induced cardiomyopathy. J. Clin. Pharm. Ther. 2019, 44, 644–646. [Google Scholar] [CrossRef]
- Patrick, J.H.; Tobias, J.D. Gabapentin and intraoperative hypotension. Pediatr. Anesth. Crit. Care J. 2021, 9, 59–64. [Google Scholar]
- Chen, H.-H.; Li, Y.-D.; Cheng, P.-W.; Fang, Y.-C.; Lai, C.-C.; Tseng, C.-J.; Pan, J.-Y.; Yeh, T.-C. Gabapentin reduces blood pressure and heart rate through the nucleus tractus solitarii. Acta Cardiol. Sin. 2019, 35, 627–633. [Google Scholar]
- Behuliak, M.; Bencze, M.; Polgárová, K.; Kuneš, J.; Vaněčková, I.; Zicha, J. Hemodynamic Response to Gabapentin in Conscious Spontaneously Hypertensive Rats. Role Sympathetic Nerv. Syst. 2018, 72, 676–685. [Google Scholar] [CrossRef]
- Largeau, B.; Bordy, R.; Pasqualin, C.; Bredeloux, P.; Cracowski, J.-L.; Lengellé, C.; Gras-Champel, V.; Auffret, M.; Maupoil, V.; Jonville-Béra, A.-P. Gabapentinoid-induced peripheral edema and acute heart failure: A translational study combining pharmacovigilance data and in vitro animal experiments. Biomed. Pharmacother. 2022, 149, 112807. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Veronezi, T.M.; Lopes, D.J.; Zardo, I.L.; Ferronatto, J.V.; Trojan, M.M.; Franck, K.R.; de Azevedo, A.F.; Spiering, A.G.; Nunes, L.N.; Fadel, L.; et al. Evaluation of the effects of gabapentin on the physiologic and echocardiographic variables of healthy cats: A prospective, randomized and blinded study. J. Feline Med. Surg. 2022, 24, e498–e504. [Google Scholar] [CrossRef]
- Vetter, S.W.; Leclerc, E. Novel aspects of CaM target recognition and activation. Eur. J. Biochem. 2003, 270, 404–414. [Google Scholar] [CrossRef]
- Allen, M.E.; LeBlanc, N.L.; Scollan, K.F. Hemodynamic, echocardiographic, and sedative effects of oral gabapentin in healthy Cats. J. Am. Anim. Hosp. Assoc. 2021, 57, 278–284. [Google Scholar] [CrossRef]
- Page, R.L.; Cantu, M.; Lindenfeld, J.; Hergott, L.J.; Lowes, B.D. Possible heart failure exacerbation associated with pregabalin: Case discussion and literature review. J. Cardiovasc. Med. 2008, 9, 922–925. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, R.H.; Jaarsma, T.; Van Den Broek, S.A.; Haaijer-Ruskamp, F.M. Decompensation of chronic heart failure associated with pregabalin in a 73-year-old patient with postherpetic neuralgia: A case report. Br. J. Clin. Pharmacol. 2008, 66, 327. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Mockler, M.; Ryder, M.; Ledwidge, M.; McDonald, K. Decompensation of chronic heart failure associated with pregabalin in patients with neuropathic pain. J. Card. Fail. 2007, 13, 227–229. [Google Scholar] [CrossRef]
- Rasimas, J.J.; Burkhart, K.K. Cardiac conduction disturbances after an overdose of nefazodone and gabapentin. Am. J. Emerg. Med. 2006, 24, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Aksakal, E.; Bakirci, E.M.; Emet, M.; Uzkeser, M. Complete atrioventricular block due to overdose of pregabalin. Am. J. Emerg. Med. 2012, 30, 2101.e1–2101.e4. [Google Scholar] [CrossRef]
- Klein-Schwartz, W.; Shepherd, J.G.; Gorman, S.; Dahl, B. Characterization of gabapentin overdose using a poison center case series. J. Toxicol. Clin. Toxicol. 2003, 41, 11–15. [Google Scholar] [CrossRef]
- Taylor, C.P.; Gee, N.S.; Su, T.-Z.; Kocsis, J.D.; Welty, D.F.; Brown, J.P.; Dooley, D.J.; Boden, P.; Singh, L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998, 29, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wu, H.; Yu, X.; Song, T.; Xu, X.; Xu, F. The calcium channel α2δ1 subunit: Interactional targets in primary sensory neurons and role in neuropathic pain. Front. Cell. Neurosci. 2021, 15, 699731. [Google Scholar] [CrossRef]
- Lanzetti, S.; Di Biase, V. Small molecules as modulators of voltage-gated calcium channels in neurological disorders: State of the art and perspectives. Molecules 2022, 27, 1312. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef]
- Chincholkar, M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: A narrative review. Br. J. Anaesth. 2018, 120, 1315–1334. [Google Scholar] [CrossRef]
- Fuller-Bicer, G.A.; Varadi, G.; Koch, S.E.; Ishii, M.; Bodi, I.; Kadeer, N.; Muth, J.N.; Mikala, G.; Petrashevskaya, N.N.; Jordan, M.A.; et al. Targeted disruption of the voltage-dependent calcium channel alpha2/delta-1-subunit. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H117–H124. [Google Scholar] [CrossRef]
- Roden, D.M.; Balser, J.R.; George, A.L., Jr.; Anderson, M.E. Cardiac ion channels. Annu. Rev. Physiol. 2002, 64, 431–475. [Google Scholar] [CrossRef]
- Eisner, D. Calcium in the heart: From physiology to disease. Exp. Physiol. 2014, 99, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.S.; Bers, D.M. Role of Ca2+/CaM-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc. Res. 2007, 73, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Fabiato, A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983, 245, C1–C14. [Google Scholar] [CrossRef]
- Otsuka, M.; Ebashi, F.; Imai, S. Cardiac Myosin B and Calcium Ions. J. Biochem. 1964, 55, 192–194. [Google Scholar] [PubMed]
- Tang, W.; Sencer, S.; Hamilton, S.L. CaM modulation of proteins involved in excitation-contraction coupling. Front. Biosci.-Landmark 2002, 7, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Nakai, J.; Sekiguchi, N.; Rando, T.A.; Allen, P.D.; Beam, K.G. Two regions of the ryanodine receptor involved in coupling with L-type Ca2+ channels. J. Biol. Chem. 1998, 273, 13403–13406. [Google Scholar] [CrossRef] [PubMed]
- Nakai, J.; Dirksen, R.T.; Nguyen, H.T.; Pessah, I.N.; Beam, K.G.; Allen, P.D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 1996, 380, 72–75. [Google Scholar] [CrossRef]
- Zhang, T.; Brown, J.H. Role of Ca2+/CaM-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc. Res. 2004, 63, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Schulman, H.; Anderson, M.E. Ca/CaM-dependent Protein Kinase II in Heart Failure. Drug Discov. Today Dis. Mech. 2010, 7, e117–e122. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Before GBP (Baseline) | After GBP (at 30 min) |
|---|---|---|
| MAP (mmHg) | 90.57 ± 21.27 | 52.88 ± 6.65 ** |
| SP (mmHg) | 111.46 ± 22.46 | 68.88 ± 7.37 ** |
| DP (mmHg) | 73.49 ± 20.68 | 40.35 ± 6.47 * |
| PP (mmHg) | 37.96 ± 2.02 | 28.52 ± 3.23 ** |
| HR (bpm) | 369.22 ± 53.71 | 299.87 ± 31.68 * |
| T-to-P (s) | 0.035 ± 0.005 | 0.047 ± 0.006 * |
| LVPmax (mmHg) | 118.01 ± 23.21 | 85.69 ± 4.64 * |
| LVPmin (mmHg) | −1.88 ± 4.63 | −2.04 ± 4.81 ns |
| LVEDP (mmHg) | 6.03 ± 4.46 | 4.91 ± 3.99 ns |
| dP/dtmax (mmHg/s) | 7746.76 ± 2022.69 | 4919.22 ± 919.05 * |
| dP/dtmin (mmHg/s) | −8822.36 ± 2783.16 | −4662.46 ± 677.01 * |
| CI (1/s) | 112.44 ± 12.58 | 108.46 ± 15.66 ns |
| PTI (mmHg.s) | 7.52 ± 0.94 | 6.01 ± 0.15 ns |
| IRP-AdP/dt (mmHg/s) | −4610.35 ± 936.66 | −2762.92 ± 262.55 * |
| Tau (s) | 0.0129 ± 0.0025 | 0.0153 ± 0.0022 * |
| SD (s) | 0.081 ± 0.007 | 0.091 ± 0.009 * |
| DD (s) | 0.085 ± 0.017 | 0.111 ± 0.015 * |
| CD (s) | 0.165 ± 0.024 | 0.201 ± 0.024 * |
| Parameters | Saline-Rats | GBP-Rats |
|---|---|---|
| MAP (mmHg) | 93.49 ± 3.28 | 73.96 ± 11.83 ** |
| SP (mmHg) | 114.33 ± 3.24 | 94.01 ± 12.93 ** |
| DP (mmHg) | 76.47 ± 3.79 | 58.02 ± 11.22 ** |
| PP (mmHg) | 37.86 ± 3.22 | 35.99 ± 2.38 ns |
| HR (bpm) | 383.15 ± 26.24 | 317.89 ± 12.12 ** |
| T-to-P (s) | 0.032 ± 0.001 | 0.042 ± 0.004 *** |
| LVPmax (mmHg) | 120.68 ± 15.91 | 93.06 ± 8.48 ** |
| LVPmin (mmHg) | 3.26 ± 8.65 | −3.49 ± 1.16 ns |
| LVEDP (mmHg) | 7.77 ± 6.73 | 5.18 ± 2.62 ns |
| dP/dtmax (mmHg/s) | 7692.31 ± 1672.35 | 5527.34 ± 884.97 * |
| dP/dtmin (mmHg/s) | −8822.36 ± 3508.77 | −6011.48 ± 776.33 ns |
| CI (1/s) | 112.55 ± 12.02 | 108.65 ± 3.59 ns |
| PTI (mmHg.s) | 7.75 ± 0.49 | 6.39 ± 0.56 ns |
| IRP-AdP/dt (mmHg/s) | −3687.47 ± 1722.11 | −3455.91 ± 386.52 ns |
| Tau (s) | 0.0154 ± 0.0045 | 0.0147 ± 0.0008 ns |
| SD (s) | 0.079 ± 0.005 | 0.086 ± 0.002 * |
| DD (s) | 0.089 ± 0.019 | 0.107 ± 0.007 ns |
| CD (s) | 0.168 ± 0.024 | 0.193 ± 0.007 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pendyala, V.V.; Pribil, S.; Schaal, V.; Sharma, K.; Jagadesan, S.; Yu, L.; Kumar, V.; Guda, C.; Gao, L. Effects of Acute and Chronic Gabapentin Treatment on Cardiovascular Function of Rats. Cells 2023, 12, 2705. https://doi.org/10.3390/cells12232705
Pendyala VV, Pribil S, Schaal V, Sharma K, Jagadesan S, Yu L, Kumar V, Guda C, Gao L. Effects of Acute and Chronic Gabapentin Treatment on Cardiovascular Function of Rats. Cells. 2023; 12(23):2705. https://doi.org/10.3390/cells12232705
Chicago/Turabian StylePendyala, Ved Vasishtha, Sarah Pribil, Victoria Schaal, Kanika Sharma, Sankarasubramanian Jagadesan, Li Yu, Vikas Kumar, Chittibabu Guda, and Lie Gao. 2023. "Effects of Acute and Chronic Gabapentin Treatment on Cardiovascular Function of Rats" Cells 12, no. 23: 2705. https://doi.org/10.3390/cells12232705