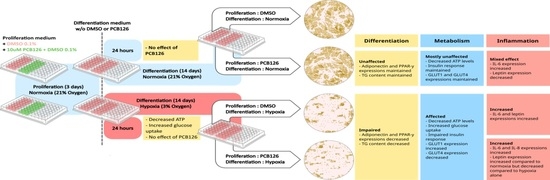
Human Preadipocytes Differentiated under Hypoxia following PCB126 Exposure during Proliferation: Effects on Differentiation, Glucose Uptake and Adipokine Profile
Abstract
:1. Introduction
2. Methods
2.1. Cell Culture
2.2. PCB126 and Hypoxia Exposure
2.3. Cell Expansion and Viability
2.4. Glucose Uptake
2.5. TG Content
2.6. Gene Expression
2.7. Statistical Analyses
3. Results
3.1. Effects of Treatments on Cell Viability and Growth
3.2. Gene Expression
3.3. TG Content and Glucose Uptake
4. Discussion
4.1. Effects of PCB126 Exposure on Human Preadipocytes under Normoxia
4.2. Effects of Hypoxia on Differentiating Human Preadipocytes
4.3. Interaction between Hypoxia and PCB126 on Differentiating Preadipocytes
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Wu, Y.; Fried, S.K. Adipose tissue remodeling in pathophysiology of obesity. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Dirinck, E.; Jorens, P.G.; Covaci, A.; Geens, T.; Roosens, L.; Neels, H. Obesity and Persistent Organic Pollutants: Possible Obesogenic Effect of Organochlorine Pesticides and Polychlorinated Biphenyls. Obesity 2011, 19, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Myre, M.; Imbeault, P. Persistent organic pollutants meet adipose tissue hypoxia: Does cross-talk contribute to inflammation during obesity? Obes. Rev. 2014, 15, 19–28. [Google Scholar] [CrossRef]
- Trayhurn, P. Hypoxia and Adipocyte Physiology: Implications for Adipose Tissue Dysfunction in Obesity. Annu. Rev. Nutr. 2014, 34, 207–236. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, K.; Jacobs, D.R., Jr.; Lee, D. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes. Rev. 2017, 18, 129–139. [Google Scholar] [CrossRef]
- Merrill, M.L.; Emond, C.; Kim, M.J.; Antignac, J.; Bizec, B.L.; Clément, K.; Birnbaum, L.S.; Barouki, R. Review Toxicological Function of Adipose Tissue: Focus on Persistent Organic. Environ. Health Perspect. 2013, 162, 162–169. [Google Scholar] [CrossRef]
- Denison, M.S.; Soshilov, A.A.; He, G.; Degroot, D.E.; Zhao, B. Exactly the Same but Different: Promiscuity and Diversity in the Molecular Mechanisms of Action of the Aryl Hydrocarbon (Dioxin) Receptor. Toxicol. Sci. 2011, 124, 1–22. [Google Scholar] [CrossRef]
- Shan, Q.; Li, H.; Chen, N.; Qu, F.; Guo, J. Understanding the Multiple Effects of PCBs on Lipid Metabolism. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3691–3702. [Google Scholar] [CrossRef]
- Nadal, A.; Quesada, I.; Tudurí, E.; Nogueiras, R.; Alonso-Magdalena, P. Endocrine-disrupting chemicals and the regulation of energy balance. Nat. Rev. Endocrinol. 2017, 13, 536–546. [Google Scholar] [CrossRef]
- Chen, C.; Brodie, A.E.; Hu, C.Y.; Brodie, A.N.N.E.; Yuan, C. CCAATEnhancer-Binding Protein p is not Affected by Tetrachlorodibenzo-p-dioxin Preadipocyte Differentiation. Obes. Res. 1997, 5, 146–152. [Google Scholar] [CrossRef]
- Gadupudi, G.; Gourronc, F.A.; Ludewig, G.; Robertson, L.W.; Klingelhutz, A.J. PCB126 Inhibits Adipogenesis of Human Preadipocytes. Toxicol. Vitr. 2015, 29, 132–141. [Google Scholar] [CrossRef]
- Phillips, M.; Enan, E.; Liu, P.C.C.; Matsumura, F. Inhibition of 3T3-L1 adipose differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Cell Sci. 1995, 108, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Gourronc, F.A.; Robertson, L.W.; Klingelhutz, A.J. A Delayed Proinflammatory Response of Human Preadipocytes to PCB126 Is Dependent on the Aryl Hydrocarbon Receptor. Environ. Sci. Pollut. Res. Int. 2018, 25, 16481. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and Adipose Tissue Function and Dysfunction in Obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef]
- Netzer, N.; Gatterer, H.; Faulhaber, M.; Burtscher, M.; Pramsohler, S.; Pesta, D. Hypoxia, Oxidative Stress and Fat. Biomolecules 2015, 5, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- El Amine, Z.; Mauger, J.F.; Imbeault, P. CYP1A1, VEGFA and Adipokine Responses of Human Adipocytes Co-exposed to PCB126 and Hypoxia. Cells 2022, 11, 2282. [Google Scholar] [CrossRef]
- Grosfeld, A.; Zilberfarb, V.; Turban, S.; André, J.; Guerre-Millo, M.; Issad, T. Hypoxia increases leptin expression in human PAZ6 adipose cells. Diabetologia 2002, 45, 527–530. [Google Scholar] [CrossRef]
- Wang, B.; Wood, I.S.; Trayhurn, P. Dysregulation of the Expression and Secretion of Inflammation-Related Adipokines by Hypoxia in Human Adipocytes. Pflug. Arch. Eur. J. Physiol. 2007, 455, 479–492. [Google Scholar] [CrossRef]
- Wang, B.; Wood, I.S.; Trayhurn, P. Hypoxia induces leptin gene expression and secretion in human preadipocytes: Differential effects of hypoxia on adipokine expression by preadipocytes. J. Endocrinol. 2008, 198, 127–134. [Google Scholar] [CrossRef]
- Mahat, B.; Mauger, J.F.; Imbeault, P. Effects of different oxygen tensions on differentiated human preadipocytes lipid storage and mobilisation. Arch. Physiol. Biochem. 2021, 127, 37–43. [Google Scholar] [CrossRef]
- O’Rourke, R.W.; Meyer, K.A.; Gaston, G.; White, A.E.; Lumeng, C.N.; Marks, D.L. Hexosamine Biosynthesis Is a Possible Mechanism Underlying Hypoxia’s Effects on Lipid Metabolism in Human Adipocytes. PLoS ONE 2013, 8, e71165. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.S.; Wang, B.; Lorente-Cebrián, S.; Trayhurn, P. Hypoxia increases expression of selective facilitative glucose transporters (GLUT) and 2-deoxy-d-glucose uptake in human adipocytes. Biochem. Biophys. Res. Commun. 2007, 361, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.S.; Stezhka, T.; Trayhurn, P. Modulation of adipokine production, glucose uptake and lactate release in human adipocytes by small changes in oxygen tension. Pflug. Arch. Eur. J. Physiol. 2011, 462, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Famulla, S.; Horrighs, A.; Cramer, A.; Sell, H.; Eckel, J. Hypoxia reduces the response of human adipocytes towards TNFα resulting in reduced NF-κB signaling and MCP-1 secretion. Int. J. Obes. 2012, 36, 986–992. [Google Scholar] [CrossRef]
- Mazzatti, D.; Lim, F.-L.; Stuart Wood, I.; Trayhurn, P. A microarray analysis of the hypoxia-induced modulation of gene expression in human adipocytes. Arch. Physiol. Biochem. 2012, 118, 112–120. [Google Scholar] [CrossRef]
- Lin, Q.; Lee, Y.J.; Yun, Z. Differentiation Arrest by Hypoxia. J. Biol. Chem. 2006, 281, 30678–30683. [Google Scholar] [CrossRef]
- Sheng, X.; Tucci, J.; Malvar, J.; Mittelman, S.D. Adipocyte differentiation is affected by media height above the cell layer. Int. J. Obes. 2013, 38, 315–320. [Google Scholar] [CrossRef]
- Weiszenstein, M.; Musutova, M.; Plihalova, A.; Westlake, K.; Elkalaf, M.; Koc, M.; Prochazka, A.; Pala, J.; Gulati, S.; Trnka, J.; et al. Adipogenesis, lipogenesis and lipolysis is stimulated by mild but not severe hypoxia in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2016, 478, 727–732. [Google Scholar] [CrossRef]
- Vorrink, S.U.; Domann, F.E. Regulatory crosstalk and interference between the xenobiotic and hypoxia sensing pathways at the AhR-ARNT-HIF1α signaling node. Chem. Biol. Interact. 2014, 218, 82–88. [Google Scholar] [CrossRef]
- Reckzeh, E.S.; Waldmann, H. Development of Glucose Transporter (GLUT) Inhibitors. Eur. J. Org. Chem. 2019, 16, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Chandramouli, V.; Carter, J.R. Metabolic effects of 2-deoxy-D-glucose in isolated fat cells. Biochim. Biophys. Acta-Gen. Subj. 1977, 496, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, E45. [Google Scholar] [CrossRef] [PubMed]
- Arsenescu, V.; Arsenescu, R.I.; King, V.; Swanson, H.; Cassis, L.A. Polychlorinated Biphenyl-77 Induces Adipocyte Differentiation and Proinflammatory Adipokines and Promotes Obesity and Atherosclerosis. Environ. Health Perspect. 2008, 116, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Shoemaker, R.; English, V.; Larian, N.; Sunkara, M.; Morris, A.J.; Walker, M.; Yiannikouris, F.; Cassis, L.A. Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environ. Health Perspect. 2015, 123, 944–950. [Google Scholar] [CrossRef]
- Kim, M.J.; Pelloux, V.; Guyot, E.; Tordjman, J.; Bui, L.; Chevallier, A.; Forest, C.; Benelli, C.; Clément, K.; Barouki, R. Inflammatory Pathway Genes Belong to Major Targets of Persistent Organic Pollutants in Adipose Cells. Environ. Health Perspect. 2012, 120, 508–514. [Google Scholar] [CrossRef]
- Lee, J.H.; Wada, T.; Febbraio, M.; He, J.; Matsubara, T.; Lee, M.J.; Gonzalez, F.J.; Xie, W. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology 2010, 139, 653. [Google Scholar] [CrossRef]
- Barouki, R.; Antignac, J.-P.; Emond, C.; Clément, K.; Birnbaum, L.; La Merrill, M.; Kim, M.J. Adipose Tissue Pollutants And Obesity. In The ECOG’s eBook on Child and Adolescent Obesity; APRIFEL Agency for the Research and Information on Fruit and Vegetables: Paris, France, 2015; pp. 1–22. [Google Scholar]
- Loiola, R.A.; Maria Dos Anjos, F.; Shimada, A.L.; Soares Cruz, W.; Drewes, C.C.; Fernandes Rodrigues, S.; Cardozo, K.H.M.; Carvalho, V.M.; Pinto, E.; Farsky, S.H. Long-term in vivo polychlorinated biphenyl 126 exposure induces oxidative stress and alters proteomic profile on islets of Langerhans. Sci. Rep. 2016, 6, 27882. [Google Scholar] [CrossRef]
- Behan-Bush, R.M.; Liszewski, J.N.; Schrodt, M.V.; Vats, B.; Li, X.; Lehmler, H.J.; Klingelhutz, A.J.; Ankrum, J.A. Toxicity Impacts on Human Adipose Mesenchymal Stem/Stromal Cells Acutely Exposed to Aroclor and Non-Aroclor Mixtures of Polychlorinated Biphenyl. Environ. Sci. Technol. 2023, 57, 1731–1742. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Ellero, S.; Chakhtoura, G.; Barreau, C.; Langouët, S.; Benelli, C.; Penicaud, L.; Beaune, P.; De Waziers, I. Xenobiotic-metabolizing cytochromes P450 in human white adipose tissue: Expression and induction. Drug Metab. Dispos. 2010, 38, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Dungen, M.W.; Murk, A.J.; Kok, D.E.; Steegenga, W.T. Toxicology in Vitro Persistent organic pollutants alter DNA methylation during human adipocyte differentiation. Toxicol. Vitr. 2017, 40, 79–87. [Google Scholar] [CrossRef]
- Liu, D.; Perkins, J.T.; Petriello, M.C.; Hennig, B. Exposure to coplanar PCBs induces endothelial cell inflammation through epigenetic regulation of NF-κB subunit p65. Toxicol. Appl. Pharmacol. 2015, 289, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Petriello, M.C.; Beibei, Z.; Bernhard Hennig, B. PCB 126 Induces Monocyte/Macrophage Polarization and Inflammation through AhR and NF-κB pathways. Physiol. Behav. 2019, 176, 139–148. [Google Scholar] [CrossRef]
- Uchi, H.; Yasukawa, F.; Furue, M. Adipokine profile of Yusho patients. Fukuoka Igaku Zasshi 2013, 104, 85–87. Available online: https://pubmed.ncbi.nlm.nih.gov/23858783/ (accessed on 4 March 2023).
- Lindén, J.; Korkalainen, M.; Lensu, S.; Tuomisto, J.; Pohjanvirta, R. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and leptin on hypothalamic mRNA expression of factors participating in food intake regulation in a TCDD-sensitive and a TCDD-resistant rat strain. J. Biochem. Mol. Toxicol. 2005, 19, 139–148. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef]
- Olsen, H.; Enan, E.; Matsumura, F. Regulation of glucose transport in the NIH 3T3 L1 preadipocyte cell line by TCDD. Environ. Health Perspect. 1994, 102, 454–458. [Google Scholar] [CrossRef]
- Enan, E.; Liu, P.C.; Matsumura, F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes reduction of glucose transporting activities in the plasma membranes of adipose tissue and pancreas from the guinea pig. J. Biol. Chem. 1992, 267, 197–202. [Google Scholar] [CrossRef]
- Nebert, D.W.; Dalton, T.P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat. Rev. Cancer 2006, 6, 947–960. [Google Scholar] [CrossRef]
- Chehade, L.; Khouri, H.; Malatier-Ségard, J.; Caron, A.; Mauger, J.F.; Chapados, N.A.; Aguer, C. Acute Exposure to Environmentally Relevant Levels of DDT Alters Muscle Mitochondrial Function in Vivo in Rats but Not in Vitro in L6 Myotubes: A Pilot Study. Toxicol. Rep. 2022, 9, 487–498. [Google Scholar] [CrossRef]
- Kim, S.A.; Lee, H.; Park, S.M.; Kim, M.J.; Lee, Y.M.; Yoon, Y.R.; Lee, H.K.; Moon, H.B.; Lee, I.K.; Lee, D.H. Effect of Low-Dose Persistent Organic Pollutants on Mitochondrial Function: Human and in Vitro Evidence. Diabetes Metab. J. 2022, 46, 592–604. [Google Scholar] [CrossRef]
- Rainey, N.E.; Saric, A.; Leberre, A.; Dewailly, E.; Slomianny, C.; Vial, G.; Zeliger, H.I.; Petit, P.X. Synergistic Cellular Effects Including Mitochondrial Destabilization, Autophagy and Apoptosis Following Low-Level Exposure to a Mixture of Lipophilic Persistent Organic Pollutants. Sci. Rep. 2017, 7, 1–20. [Google Scholar] [CrossRef]
- Gu, C.; Jun, J.C. Does Hypoxia Decrease the Metabolic Rate? Front. Endocrinol. 2018, 9, 668. [Google Scholar] [CrossRef]
- Hayashi, K.; Ochiai, T.; Ishinoda, Y.; Okamoto, T.; Maruyama, T.; Tsuda, K.; Tsubouchi, H. Relationship between cellular ATP content and cellular functions of primary cultured rat hepatocytes in hypoxia. J. Gastroenterol. Hepatol. 1997, 12, 249–256. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yokokawa, T.; Endo, Y.; Iwanaka, N.; Higashida, K.; Taguchi, S. Modest hypoxia significantly reduces triglyceride content and lipid droplet size in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2013, 440, 43–49. [Google Scholar] [CrossRef]
- Chaiban, J.T.; Bitar, F.F.; Azar, S.T. Effect of chronic hypoxia on leptin, insulin, adiponectin, and ghrelin. Metabolism 2008, 57, 1019–1022. [Google Scholar] [CrossRef]
- Yamazaki, K.; Suzuki, M.; Itoh, T.; Yamamoto, K.; Kanemitsu, M.; Matsumura, C.; Nakano, T.; Sakaki, T.; Fukami, Y.; Imaishi, H.; et al. Structural basis of species differences between human and experimental animal CYP1A1s in metabolism of 3,3′,4,4′,5-pentachlorobiphenyl. J. Biochem. 2011, 149, 487–494. [Google Scholar] [CrossRef]
- Bourez, S.; Joly, A.; Covaci, A.; Remacle, C.; Larondelle, Y.; Schneider, Y.-J.; Debier, C. Accumulation capacity of primary cultures of adipocytes for PCB-126: Influence of cell differentiation stage and triglyceride levels. Toxicol. Lett. 2012, 214, 243–250. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Amine, Z.; Mauger, J.-F.; Imbeault, P. Human Preadipocytes Differentiated under Hypoxia following PCB126 Exposure during Proliferation: Effects on Differentiation, Glucose Uptake and Adipokine Profile. Cells 2023, 12, 2326. https://doi.org/10.3390/cells12182326
El Amine Z, Mauger J-F, Imbeault P. Human Preadipocytes Differentiated under Hypoxia following PCB126 Exposure during Proliferation: Effects on Differentiation, Glucose Uptake and Adipokine Profile. Cells. 2023; 12(18):2326. https://doi.org/10.3390/cells12182326
Chicago/Turabian StyleEl Amine, Zeinab, Jean-François Mauger, and Pascal Imbeault. 2023. "Human Preadipocytes Differentiated under Hypoxia following PCB126 Exposure during Proliferation: Effects on Differentiation, Glucose Uptake and Adipokine Profile" Cells 12, no. 18: 2326. https://doi.org/10.3390/cells12182326