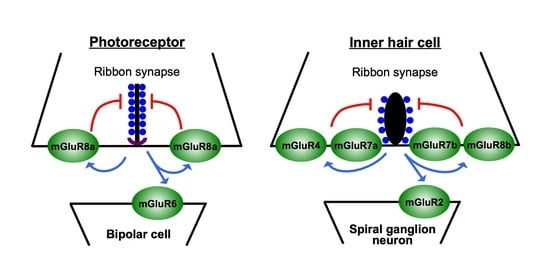
Metabotropic Glutamate Receptors at Ribbon Synapses in the Retina and Cochlea
Abstract
:1. Ribbon Synapses
2. Metabotropic Glutamate Receptors
3. Metabotropic Glutamate Receptors at Ribbon Synapses in the Retina
4. Metabotropic Glutamate Receptors at Ribbon Synapses in the Cochlea
5. Pre-Synaptic Metabotropic Glutamate Receptors Are Central Elements of Inhibitory Feed-Back Loops at Ribbon Synapses
6. Protein Interactions of Metabotropic Glutamate Receptors at Ribbon Synapses
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voorn, R.A.; Vogl, C. Molecular Assembly and Structural Plasticity of Sensory Ribbon Synapses-A Presynaptic Perspective. Int. J. Mol. Sci. 2020, 21, 8758. [Google Scholar] [CrossRef]
- Moser, T.; Grabner, C.P.; Schmitz, F. Sensory Processing at Ribbon Synapses in the Retina and the Cochlea. Physiol. Rev. 2020, 100, 103–144. [Google Scholar] [CrossRef] [PubMed]
- Baden, T.; Euler, T.; Weckstrom, M.; Lagnado, L. Spikes and ribbon synapses in early vision. Trends Neurosci. 2013, 36, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M. Electron microscopy of the inner plexiform layer of the retina in the cat and the pigeon. J. Anat. 1962, 96, 179–187. [Google Scholar]
- De Robertis, E.; Franchi, C.M. Electron microscope observations on synaptic vesicles in synapses of the retinal rods and cones. J Biophys. Biochem. Cytol. 1956, 2, 307–318. [Google Scholar] [CrossRef]
- Schmitz, F.; Konigstorfer, A.; Südhof, T.C. RIBEYE, a component of synaptic ribbons: A protein’s journey through evolution provides insight into synaptic ribbon function. Neuron 2000, 28, 857–872. [Google Scholar] [CrossRef] [Green Version]
- Chinnadurai, G. The transcriptional corepressor CtBP: A foe of multiple tumor suppressors. Cancer Res. 2009, 69, 731–734. [Google Scholar] [CrossRef] [Green Version]
- Lagnado, L.; Schmitz, F. Ribbon Synapses and Visual Processing in the Retina. Annu. Rev. Vis. Sci. 2015, 1, 235–262. [Google Scholar] [CrossRef]
- Jean, P.; Lopez de la Morena, D.; Michanski, S.; Jaime Tobon, L.M.; Chakrabarti, R.; Picher, M.M.; Neef, J.; Jung, S.; Gultas, M.; Maxeiner, S.; et al. The synaptic ribbon is critical for sound encoding at high rates and with temporal precision. Elife 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Maxeiner, S.; Luo, F.; Tan, A.; Schmitz, F.; Sudhof, T.C. How to make a synaptic ribbon: RIBEYE deletion abolishes ribbons in retinal synapses and disrupts neurotransmitter release. EMBO J. 2016, 35, 1098–1114. [Google Scholar] [CrossRef] [Green Version]
- Grabner, C.P.; Moser, T. The mammalian rod synaptic ribbon is essential for Cav channel facilitation and ultrafast synaptic vesicle fusion. Elife 2021, 10. [Google Scholar] [CrossRef]
- Fairless, R.; Williams, S.K.; Katiyar, R.; Maxeiner, S.; Schmitz, F.; Diem, R. ERG Responses in Mice with Deletion of the Synaptic Ribbon Component RIBEYE. Investig. Ophthalmol. Vis. Sci. 2020, 61, 37. [Google Scholar] [CrossRef]
- Becker, L.; Schnee, M.E.; Niwa, M.; Sun, W.; Maxeiner, S.; Talaei, S.; Kachar, B.; Rutherford, M.A.; Ricci, A.J. The presynaptic ribbon maintains vesicle populations at the hair cell afferent fiber synapse. Elife 2018, 7. [Google Scholar] [CrossRef]
- Gundelfinger, E.D.; Reissner, C.; Garner, C.C. Role of Bassoon and Piccolo in Assembly and Molecular Organization of the Active Zone. Front. Synaptic Neurosci. 2015, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- tom Dieck, S.; Altrock, W.D.; Kessels, M.M.; Qualmann, B.; Regus, H.; Brauner, D.; Fejtova, A.; Bracko, O.; Gundelfinger, E.D.; Brandstätter, J.H. Molecular dissection of the photoreceptor ribbon synapse: Physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J. Cell Biol. 2005, 168, 825–836. [Google Scholar] [CrossRef] [Green Version]
- Dick, O.; tom Dieck, S.; Altrock, W.D.; Ammermuller, J.; Weiler, R.; Garner, C.C.; Gundelfinger, E.D.; Brandstätter, J.H. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron 2003, 37, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Khimich, D.; Nouvian, R.; Pujol, R.; Tom Dieck, S.; Egner, A.; Gundelfinger, E.D.; Moser, T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature 2005, 434, 889–894. [Google Scholar] [CrossRef]
- Müller, T.M.; Gierke, K.; Joachimsthaler, A.; Sticht, H.; Izsvak, Z.; Hamra, F.K.; Fejtova, A.; Ackermann, F.; Garner, C.C.; Kremers, J.; et al. A Multiple Piccolino-RIBEYE Interaction Supports Plate-Shaped Synaptic Ribbons in Retinal Neurons. J. Neurosci. 2019, 39, 2606–2619. [Google Scholar] [CrossRef] [Green Version]
- Regus-Leidig, H.; Ott, C.; Lohner, M.; Atorf, J.; Fuchs, M.; Sedmak, T.; Kremers, J.; Fejtova, A.; Gundelfinger, E.D.; Brandstätter, J.H. Identification and immunocytochemical characterization of Piccolino, a novel Piccolo splice variant selectively expressed at sensory ribbon synapses of the eye and ear. PLoS ONE 2013, 8, e70373. [Google Scholar] [CrossRef]
- Regus-Leidig, H.; Fuchs, M.; Lohner, M.; Leist, S.R.; Leal-Ortiz, S.; Chiodo, V.A.; Hauswirth, W.W.; Garner, C.C.; Brandstätter, J.H. In vivo knockdown of Piccolino disrupts presynaptic ribbon morphology in mouse photoreceptor synapses. Front. Cell. Neurosci. 2014, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Moser, T.; Brandt, A.; Lysakowski, A. Hair cell ribbon synapses. Cell Tissue Res. 2006, 326, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Safieddine, S.; El-Amraoui, A.; Petit, C. The auditory hair cell ribbon synapse: From assembly to function. Annu. Rev. Neurosci. 2012, 35, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, T. Ribbon synapses in zebrafish hair cells. Hear. Res 2015, 330, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichmann, C.; Moser, T. Relating structure and function of inner hair cell ribbon synapses. Cell Tissue Res. 2015, 361, 95–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Lin, Z.; An, Y.; Lin, J.; Zhang, A.; Wang, S.; Tu, H.; Ran, J.; Wang, J.; Liang, Y.; et al. Piccolo is essential for the maintenance of mouse retina but not cochlear hair cell function. Aging 2021, 13, 11678–11695. [Google Scholar] [CrossRef] [PubMed]
- Thoreson, W.B. Transmission at rod and cone ribbon synapses in the retina. Pflug. Arch. 2021, 473, 1469–1491. [Google Scholar] [CrossRef]
- Matthews, G.; Fuchs, P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat. Rev. Neurosci. 2010, 11, 812–822. [Google Scholar] [CrossRef]
- Regus-Leidig, H.; Brandstätter, J.H. Structure and function of a complex sensory synapse. Acta Physiol. 2012, 204, 479–486. [Google Scholar] [CrossRef]
- Heidelberger, R.; Thoreson, W.B.; Witkovsky, P. Synaptic transmission at retinal ribbon synapses. Prog. Retin. Eye Res. 2005, 24, 682–720. [Google Scholar] [CrossRef] [Green Version]
- Frank, T.; Rutherford, M.A.; Strenzke, N.; Neef, A.; Pangrsic, T.; Khimich, D.; Fejtova, A.; Gundelfinger, E.D.; Liberman, M.C.; Harke, B.; et al. Bassoon and the synaptic ribbon organize Ca(2)+ channels and vesicles to add release sites and promote refilling. Neuron 2010, 68, 724–738. [Google Scholar] [CrossRef] [Green Version]
- Edmonds, B.W.; Gregory, F.D.; Schweizer, F.E. Evidence that fast exocytosis can be predominantly mediated by vesicles not docked at active zones in frog saccular hair cells. J. Physiol. 2004, 560, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.A. Time and intensity coding at the hair cell’s ribbon synapse. J. Physiol. 2005, 566, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Graydon, C.W.; Cho, S.; Li, G.L.; Kachar, B.; von Gersdorff, H. Sharp Ca(2)(+) nanodomains beneath the ribbon promote highly synchronous multivesicular release at hair cell synapses. J. Neurosci. 2011, 31, 16637–16650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, B.; Snellman, J.; Chen, S.; Li, W.; Zenisek, D. Synaptic ribbons influence the size and frequency of miniature-like evoked postsynaptic currents. Neuron 2013, 77, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharm. 2018, 93, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Lagerstrom, M.C.; Schioth, H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug. Discov. 2008, 7, 339–357. [Google Scholar] [CrossRef]
- Ellaithy, A.; Gonzalez-Maeso, J.; Logothetis, D.A.; Levitz, J. Structural and Biophysical Mechanisms of Class C G Protein-Coupled Receptor Function. Trends Biochem. Sci. 2020, 45, 1049–1064. [Google Scholar] [CrossRef]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, F.; Bockaert, J.; Collingridge, G.L.; Conn, P.J.; Ferraguti, F.; Schoepp, D.D.; Wroblewski, J.T.; Pin, J.P. Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology 2011, 60, 1017–1041. [Google Scholar] [CrossRef] [Green Version]
- Perroy, J.; Prezeau, L.; De Waard, M.; Shigemoto, R.; Bockaert, J.; Fagni, L. Selective blockade of P/Q-type calcium channels by the metabotropic glutamate receptor type 7 involves a phospholipase C pathway in neurons. J. Neurosci. 2000, 20, 7896–7904. [Google Scholar]
- Seebahn, A.; Dinkel, H.; Mohrlüder, J.; Hartmann, R.; Vogel, N.; Becker, C.M.; Sticht, H.; Enz, R. Structural characterization of intracellular C-terminal domains of group III metabotropic glutamate receptors. FEBS Lett. 2011, 585, 511–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enz, R. The trick of the tail: Protein-protein interactions of metabotropic glutamate receptors. Bioessays 2007, 29, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Habrian, C.H.; Levitz, J.; Vyklicky, V.; Fu, Z.; Hoagland, A.; McCort-Tranchepain, I.; Acher, F.; Isacoff, E.Y. Conformational pathway provides unique sensitivity to a synaptic mGluR. Nat. Commun. 2019, 10, 5572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doumazane, E.; Scholler, P.; Zwier, J.M.; Trinquet, E.; Rondard, P.; Pin, J.P. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 2011, 25, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Munguba, H.; Gutzeit, V.A.; Singh, D.R.; Kristt, M.; Dittman, J.S.; Levitz, J. Defining the Homo- and Heterodimerization Propensities of Metabotropic Glutamate Receptors. Cell Rep. 2020, 31, 107605. [Google Scholar] [CrossRef] [PubMed]
- Moller, T.C.; Moreno-Delgado, D.; Pin, J.P.; Kniazeff, J. Class C G protein-coupled receptors: Reviving old couples with new partners. Biophys. Rep. 2017, 3, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Z.; Lv, X.; Lin, X.; O’Brien, D.E.; Altman, M.K.; Lindsley, C.W.; Javitch, J.A.; Niswender, C.M.; Conn, P.J. Input-specific regulation of glutamatergic synaptic transmission in the medial prefrontal cortex by mGlu2/mGlu4 receptor heterodimers. Sci. Signal. 2021, 14. [Google Scholar] [CrossRef]
- Du, J.; Wang, D.; Fan, H.; Xu, C.; Tai, L.; Lin, S.; Han, S.; Tan, Q.; Wang, X.; Xu, T.; et al. Structures of human mGlu2 and mGlu7 homo- and heterodimers. Nature 2021, 594, 589–593. [Google Scholar] [CrossRef]
- McCullock, T.W.; Kammermeier, P.J. The evidence for and consequences of metabotropic glutamate receptor heterodimerization. Neuropharmacology 2021, 199, 108801. [Google Scholar] [CrossRef]
- Wässle, H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 2004, 5, 747–757. [Google Scholar] [CrossRef]
- Koulen, P.; Kuhn, R.; Wässle, H.; Brandstätter, J.H. Modulation of the intracellular calcium concentration in photoreceptor terminals by a presynaptic metabotropic glutamate receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 9909–9914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koulen, P.; Brandstätter, J.H. Pre- and Postsynaptic Sites of Action of mGluR8a in the mammalian retina. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1933–1940. [Google Scholar]
- Koulen, P.; Liu, J.; Nixon, E.; Madry, C. Interaction between mGluR8 and calcium channels in photoreceptors is sensitive to pertussis toxin and occurs via G protein betagamma subunit signaling. Investig. Ophthalmol. Vis. Sci. 2005, 46, 287–291. [Google Scholar] [CrossRef]
- Max-Planck-Society. “Sunglasses” in The Eye. ScienceDaily. 1999. Available online: www.sciencedaily.com/releases/1999/09/990910080155.htm (accessed on 24 January 2022).
- Mascia, F.; Klotz, L.; Lerch, J.; Ahmed, M.H.; Zhang, Y.; Enz, R. CRIP1a inhibits endocytosis of G-protein coupled receptors activated by endocannabinoids and glutamate by a common molecular mechanism. J. Neurochem. 2017, 141, 577–591. [Google Scholar] [CrossRef] [Green Version]
- Dunn, H.A.; Patil, D.N.; Cao, Y.; Orlandi, C.; Martemyanov, K.A. Synaptic adhesion protein ELFN1 is a selective allosteric modulator of group III metabotropic glutamate receptors in trans. Proc. Natl. Acad. Sci. USA 2018, 115, 5022–5027. [Google Scholar] [CrossRef] [Green Version]
- Dunn, H.A.; Zucca, S.; Dao, M.; Orlandi, C.; Martemyanov, K.A. ELFN2 is a postsynaptic cell adhesion molecule with essential roles in controlling group III mGluRs in the brain and neuropsychiatric behavior. Mol. Psychiatry 2019, 24, 1902–1919. [Google Scholar] [CrossRef]
- Seebahn, A.; Rose, M.; Enz, R. RanBPM is expressed in synaptic layers of the mammalian retina and binds to metabotropic glutamate receptors. FEBS Lett. 2008, 582, 2453–2457. [Google Scholar] [CrossRef] [Green Version]
- Nomura, A.; Shigemoto, R.; Nakamura, Y.; Okamoto, N.; Mizuno, N.; Nakanishi, S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell 1994, 77, 361–369. [Google Scholar] [CrossRef]
- Martemyanov, K.A. G protein signaling in the retina and beyond: The Cogan lecture. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8201–8207. [Google Scholar] [CrossRef] [Green Version]
- Zeitz, C.; Robson, A.G.; Audo, I. Congenital stationary night blindness: An analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Prog. Retin. Eye Res. 2015, 45, 58–110. [Google Scholar] [CrossRef]
- Varin, J.; Bouzidi, N.; Dias, M.M.S.; Pugliese, T.; Michiels, C.; Robert, C.; Desrosiers, M.; Sahel, J.A.; Audo, I.; Dalkara, D.; et al. Restoration of mGluR6 Localization Following AAV-Mediated Delivery in a Mouse Model of Congenital Stationary Night Blindness. Investig. Ophthalmol. Vis. Sci. 2021, 62, 24. [Google Scholar] [CrossRef]
- Brandstätter, J.H.; Koulen, P.; Kuhn, R.; van der Putten, H.; Wässle, H. Compartmental localization of a metabotropic glutamate receptor (mGluR7): Two different active sites at a retinal synapse. J. Neurosci. 1996, 16, 4749–4756. [Google Scholar] [PubMed]
- Brandstätter, J.H.; Koulen, P.; Wässle, H. Diversity of glutamate receptors in the mammalian retina. Vis. Res 1998, 38, 1385–1397. [Google Scholar] [CrossRef] [Green Version]
- Koulen, P.; Malitschek, B.; Kuhn, R.; Wässle, H.; Brandstätter, J.H. Group II and group III metabotropic glutamate receptors in the rat retina: Distributions and developmental expression patterns. Eur. J. Neurosci. 1996, 8, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, A.J. Integrating the active process of hair cells with cochlear function. Nat. Rev. Neurosci. 2014, 15, 600–614. [Google Scholar] [CrossRef]
- Michanski, S.; Smaluch, K.; Steyer, A.M.; Chakrabarti, R.; Setz, C.; Oestreicher, D.; Fischer, C.; Mobius, W.; Moser, T.; Vogl, C.; et al. Mapping developmental maturation of inner hair cell ribbon synapses in the apical mouse cochlea. Proc. Natl. Acad. Sci. USA 2019, 116, 6415–6424. [Google Scholar] [CrossRef] [Green Version]
- Ohn, T.L.; Rutherford, M.A.; Jing, Z.; Jung, S.; Duque-Afonso, C.J.; Hoch, G.; Picher, M.M.; Scharinger, A.; Strenzke, N.; Moser, T. Hair cells use active zones with different voltage dependence of Ca2+ influx to decompose sounds into complementary neural codes. Proc. Natl. Acad. Sci. USA 2016, 113, 4716–4725. [Google Scholar] [CrossRef] [Green Version]
- Fettiplace, R. Hair Cell Transduction, Tuning, and Synaptic Transmission in the Mammalian Cochlea. Compr. Physiol. 2017, 7, 1197–1227. [Google Scholar] [CrossRef] [Green Version]
- Grunert, U.; Martin, P.R. Morphology, Molecular Characterization, and Connections of Ganglion Cells in Primate Retina. Annu. Rev. Vis. Sci. 2021, 7, 73–103. [Google Scholar] [CrossRef]
- Gomez-Casati, M.E.; Goutman, J.D. Divide and conquer acoustic diversity. EMBO J 2021, 40, 107531. [Google Scholar] [CrossRef]
- Ozcete, O.D.; Moser, T. A sensory cell diversifies its output by varying Ca(2+) influx-release coupling among active zones. EMBO J. 2021, 40, 106010. [Google Scholar] [CrossRef]
- Liberman, L.D.; Wang, H.; Liberman, M.C. Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses. J. Neurosci. 2011, 31, 801–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, A.C.; Frank, T.; Khimich, D.; Hoch, G.; Riedel, D.; Chapochnikov, N.M.; Yarin, Y.M.; Harke, B.; Hell, S.W.; Egner, A.; et al. Tuning of synapse number, structure and function in the cochlea. Nat. Neurosci. 2009, 12, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Wendler, O.; Frischknecht, R.; Shigemoto, R.; Schulze, H.; Enz, R. Localization of group II and III metabotropic glutamate receptors at pre- and postsynaptic sites of inner hair cell ribbon synapses. FASEB J. 2019, 33, 13734–13746. [Google Scholar] [CrossRef] [Green Version]
- Klotz, L.; Enz, R. MGluR7 is a presynaptic metabotropic glutamate receptor at ribbon synapses of inner hair cells. FASEB J 2021, 35, e21855. [Google Scholar] [CrossRef]
- Beer-Hammer, S.; Lee, S.C.; Mauriac, S.A.; Leiss, V.; Groh, I.A.M.; Novakovic, A.; Piekorz, R.P.; Bucher, K.; Chen, C.; Ni, K.; et al. Galphai Proteins are Indispensable for Hearing. Cell Physiol. Biochem. 2018, 47, 1509–1532. [Google Scholar] [CrossRef]
- Jean, P.; Ozcete, O.D.; Tarchini, B.; Moser, T. Intrinsic planar polarity mechanisms influence the position-dependent regulation of synapse properties in inner hair cells. Proc. Natl. Acad. Sci. USA 2019, 116, 9084–9093. [Google Scholar] [CrossRef] [Green Version]
- Coate, T.M.; Scott, M.K.; Gurjar, M. Current concepts in cochlear ribbon synapse formation. Synapse 2019, 73, e22087. [Google Scholar] [CrossRef]
- Granzotto, A.; Weiss, J.; Sensi, S.L. Excitotoxicity Turns 50. The Death That Never Dies. Front. Neurosci. 2022, 15, 831809. [Google Scholar] [CrossRef]
- Hu, N.; Rutherford, M.A.; Green, S.H. Protection of cochlear synapses from noise-induced excitotoxic trauma by blockade of Ca(2+)-permeable AMPA receptors. Proc. Natl. Acad. Sci. USA 2020, 117, 3828–3838. [Google Scholar] [CrossRef]
- Friedman, R.A.; Van Laer, L.; Huentelman, M.J.; Sheth, S.S.; Van Eyken, E.; Corneveaux, J.J.; Tembe, W.D.; Halperin, R.F.; Thorburn, A.Q.; Thys, S.; et al. GRM7 variants confer susceptibility to age-related hearing impairment. Hum. Mol. Genet. 2009, 18, 785–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.; Jiao, J.; Chen, G.; Zhou, W.; Zhang, H.; Wu, H.; Li, Y.; Gu, G.; Zheng, Y.; Yu, Y.; et al. Effect of GRM7 polymorphisms on the development of noise-induced hearing loss in Chinese Han workers: A nested case-control study. BMC Med. Genet. 2018, 19, 4. [Google Scholar] [CrossRef] [Green Version]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharm. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [Green Version]
- Van Laer, L.; Huyghe, J.R.; Hannula, S.; Van Eyken, E.; Stephan, D.A.; Maki-Torkko, E.; Aikio, P.; Fransen, E.; Lysholm-Bernacchi, A.; Sorri, M.; et al. A genome-wide association study for age-related hearing impairment in the Saami. Eur. J. Hum. Genet. 2010, 18, 685–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.L.; Fisher, L.M.; Ohmen, J.; Parody, R.; Fong, C.T.; Frisina, S.T.; Mapes, F.; Eddins, D.A.; Robert Frisina, D.; Frisina, R.D.; et al. GRM7 variants associated with age-related hearing loss based on auditory perception. Hear. Res. 2012, 294, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Chang, N.C.; Dai, C.Y.; Lin, W.Y.; Yang, H.L.; Wang, H.M.; Chien, C.Y.; Hsieh, M.H.; Ho, K.Y. The Association of GRM7 Single Nucleotide Polymorphisms with Age-Related Hearing Impairment in a Taiwanese Population. J. Int. Adv. Otol. 2018, 14, 170–175. [Google Scholar] [CrossRef]
- Fisher, N.M.; Gould, R.W.; Gogliotti, R.G.; McDonald, A.J.; Badivuku, H.; Chennareddy, S.; Buch, A.B.; Moore, A.M.; Jenkins, M.T.; Robb, W.H.; et al. Phenotypic profiling of mGlu7 knockout mice reveals new implications for neurodevelopmental disorders. Genes Brain Behav. 2020, 19, e12654. [Google Scholar] [CrossRef]
- Enz, R. Structure of metabotropic glutamate receptor C-terminal domains in contact with interacting proteins. Front. Mol. Neurosci. 2012, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Levitz, J.; Habrian, C.; Bharill, S.; Fu, Z.; Vafabakhsh, R.; Isacoff, E.Y. Mechanism of Assembly and Cooperativity of Homomeric and Heteromeric Metabotropic Glutamate Receptors. Neuron 2016, 92, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Moreno Delgado, D.; Moller, T.C.; Ster, J.; Giraldo, J.; Maurel, D.; Rovira, X.; Scholler, P.; Zwier, J.M.; Perroy, J.; Durroux, T.; et al. Pharmacological evidence for a metabotropic glutamate receptor heterodimer in neuronal cells. Elife 2017, 6. [Google Scholar] [CrossRef]
- Enz, R. Metabotropic glutamate receptors and interacting proteins: Evolving drug targets. Curr. Drug Targets 2012, 13, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Enz, R. The metabotropic glutamate receptor mGluR7b binds to the catalytic gamma-subunit of protein phosphatase 1. J. Neurochem. 2002, 81, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Croci, C.; Sticht, H.; Brandstätter, J.H.; Enz, R. Group I metabotropic glutamate receptors bind to protein phosphatase 1C. Mapping and modeling of interacting sequences. J. Biol. Chem. 2003, 278, 50682–50690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiselbach, H.; Sticht, H.; Enz, R. Structural analysis of the protein phosphatase 1 docking motif: Molecular description of binding specificities identifies interacting proteins. Chem. Biol. 2006, 13, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enz, R.; Croci, C. Different binding motifs in metabotropic glutamate receptor type 7b for filamin A, protein phosphatase 1C, protein interacting with protein kinase C (PICK) 1 and syntenin allow the formation of multimeric protein complexes. Biochem. J. 2003, 372, 183–191. [Google Scholar] [CrossRef]
- Dütting, E.; Schröder-Kress, N.; Sticht, H.; Enz, R. SUMO E3 ligases are expressed in the retina and regulate SUMOylation of the metabotropic glutamate receptor 8b. Biochem. J. 2011, 435, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Blundell, T.L.; Gupta, M.N.; Hasnain, S.E. Intrinsic disorder in proteins: Relevance to protein assemblies, drug design and host-pathogen interactions. Prog. Biophys. Mol. Biol. 2020, 156, 34–42. [Google Scholar] [CrossRef]
- Bugge, K.; Brakti, I.; Fernandes, C.B.; Dreier, J.E.; Lundsgaard, J.E.; Olsen, J.G.; Skriver, K.; Kragelund, B.B. Interactions by Disorder—A Matter of Context. Front. Mol. Biosci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Muto, T.; Tsuchiya, D.; Morikawa, K.; Jingami, H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 2007, 104, 3759–3764. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, D.M.; Rasmussen, S.G.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Kukhtina, V.; Kottwitz, D.; Strauss, H.; Heise, B.; Chebotareva, N.; Tsetlin, V.; Hucho, F. Intracellular domain of nicotinic acetylcholine receptor: The importance of being unfolded. J. Neurochem. 2006, 97 (Suppl. 1), 63–67. [Google Scholar] [CrossRef]
- Sobolevsky, A.I.; Rosconi, M.P.; Gouaux, E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 2009, 462, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Warnet, X.L.; Bakke Krog, H.; Sevillano-Quispe, O.G.; Poulsen, H.; Kjaergaard, M. The C-terminal domains of the NMDA receptor: How intrinsically disordered tails affect signalling, plasticity and disease. Eur. J. Neurosci. 2021, 54, 6713–6739. [Google Scholar] [CrossRef]
- Seebahn, A.; Sticht, H.; Enz, R. Expression, purification, and structural analysis of intracellular C-termini from metabotropic glutamate receptors. Methods Enzym. 2013, 520, 257–279. [Google Scholar] [CrossRef]
- Hu, S.S.; Arnold, A.; Hutchens, J.M.; Radicke, J.; Cravatt, B.F.; Wager-Miller, J.; Mackie, K.; Straiker, A. Architecture of cannabinoid signaling in mouse retina. J. Comp. Neurol. 2010, 518, 3848–3866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, S.F.; Yazulla, S. Retrograde endocannabinoid inhibition of goldfish retinal cones is mediated by 2-arachidonoyl glycerol. Vis. Neurosci. 2007, 24, 257–267. [Google Scholar] [CrossRef]
- Furman, A.C.; Kujawa, S.G.; Liberman, M.C. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J. Neurophysiol. 2013, 110, 577–586. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klotz-Weigand, L.; Enz, R. Metabotropic Glutamate Receptors at Ribbon Synapses in the Retina and Cochlea. Cells 2022, 11, 1097. https://doi.org/10.3390/cells11071097
Klotz-Weigand L, Enz R. Metabotropic Glutamate Receptors at Ribbon Synapses in the Retina and Cochlea. Cells. 2022; 11(7):1097. https://doi.org/10.3390/cells11071097
Chicago/Turabian StyleKlotz-Weigand, Lisa, and Ralf Enz. 2022. "Metabotropic Glutamate Receptors at Ribbon Synapses in the Retina and Cochlea" Cells 11, no. 7: 1097. https://doi.org/10.3390/cells11071097