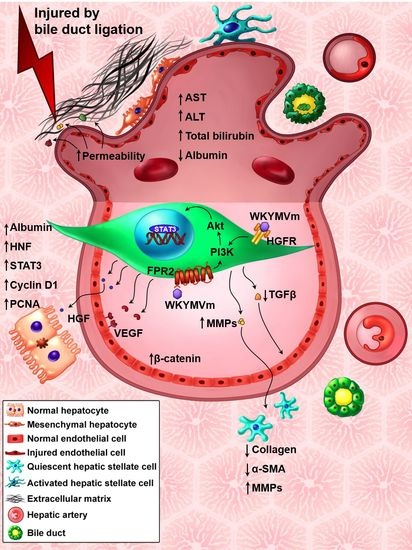
Combination Therapy of Placenta-Derived Mesenchymal Stem Cells with WKYMVm Promotes Hepatic Function in a Rat Model with Hepatic Disease via Vascular Remodeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Animals
2.4. Biochemical Analysis
2.5. Cell Proliferation Assay
2.6. Senescence-Associated β-Galactosidase (SA-β Gal) Assay
2.7. Differentiation of WKYMVm-Treated PD-MSCs
2.8. Tube Formation Assay
2.9. Dextran Permeability Assay
2.10. Histological Analysis
2.11. Immunohistochemistry
2.12. Immunofluorescence
2.13. Enzyme-Linked Immunosorbent Assay (ELISA)
2.14. Western Blot
2.15. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.16. Gelatin Zymography
2.17. Statistical Analysis
3. Results
3.1. Characterization of PD-MSCs Combined with WKYMVm
3.2. WKYMVm Enhances the Effects of PD-MSCs
3.3. PD-MSCs Combined with WKYMVm Enhance Hepatic Function in the BDL Rat Model
3.4. PD-MSCs Combined with WKYMVm Promote Vascular Regeneration in the BDL Rat Liver
3.5. PD-MSCs Combined with WKYMVm Promote Angiogenic Activity in HUVECs
3.6. PD-MSCs Combined with WKYMVm Attenuate Hepatic Fibrosis in the BDL Rat Liver
3.7. PD-MSCs Combined with WKYMVm Inhibit HSC Activation In Vitro
3.8. PD-MSCs Combined with WKYMVm Improve Hepatic Regeneration in the BDL Rat Model
3.9. PD-MSCs Combined with WKYMVm Can Regenerate Damaged Hepatocytes In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otrock, Z.K.; Mahfouz, R.A.R.; Makarem, J.A.; Shamseddine, A.I. Understanding the biology of angiogenesis: Review of the most important molecular mechanisms. Blood Cells Mol. Dis. 2007, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Praktiknjo, M.; Lehmann, J.; Nielsen, M.J.; Schierwagen, R.; Uschner, F.E.; Meyer, C.; Thomas, D.; Strassburg, C.P.; Bendtsen, F.; Møller, S.; et al. Acute decompensation boosts hepatic collagen type III deposition and deteriorates experimental and human cirrhosis. Hepatol. Commun. 2018, 2, 211–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, S.L.; Sheppard, D.; Duffield, J.S.; Violette, S. Therapy for Fibrotic Diseases: Nearing the Starting Line. Sci. Transl. Med. 2013, 5, 167sr1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Yannam, G.R.; Nishikawa, T.; Yamamoto, T.; Basma, H.; Ito, R.; Nagaya, M.; Dutta-Moscato, J.; Stolz, D.B.; Duan, F.; et al. The microenvironment in hepatocyte regeneration and function in rats with advanced cirrhosis. Hepatology 2012, 55, 1529–1539. [Google Scholar] [CrossRef] [Green Version]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.-E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, D.J.; Ginsberg, M.; Israely, E.; Palikuqi, B.; Poulos, M.G.; James, D.; Ding, B.-S.; Schachterle, W.; Liu, Y.; Rosenwaks, Z.; et al. Molecular Signatures of Tissue-Specific Microvascular Endothelial Cell Heterogeneity in Organ Maintenance and Regeneration. Dev. Cell 2013, 26, 204–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, E.; Sakisaka, S.; Matsuo, K.; Tanikawa, K.; Sata, M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J. Histochem. Cytochem. 2001, 49, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Kwon, J.; Popov, Y.; Gajdos, G.B.; Ordog, T.; Brekken, R.A.; Mukhopadhyay, D.; Schuppan, D.; Bi, Y.; Simonetto, D.; et al. Vascular Endothelial Growth Factor Promotes Fibrosis Resolution and Repair in Mice. Gastroenterology 2014, 146, 1339–1350.e1. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Ye, T.; Sun, Y.; Ji, G.; Shido, K.; Chen, Y.; Luo, L.; Na, F.; Li, X.; Huang, Z.; et al. Targeting the vascular and perivascular niches as a regenerative therapy for lung and liver fibrosis. Sci. Transl. Med. 2017, 9, eaai8710. [Google Scholar] [CrossRef] [Green Version]
- Hanai, J.-I.; Dhanabal, M.; Karumanchi, S.A.; Albanese, C.; Waterman, M.; Chan, B.; Ramchandran, R.; Pestell, R.; Sukhatme, V.P. Endostatin Causes G1 Arrest of Endothelial Cells through Inhibition of Cyclin D1. J. Biol. Chem. 2002, 277, 16464–16469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korn, C.; Scholz, B.; Hu, J.; Srivastava, K.; Wojtarowicz, J.; Arnsperger, T.; Adams, R.H.; Boutros, M.; Augustin, H.G.; Augustin, I. Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development 2014, 141, 1757–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-D.; Kim, S.-S.; Cha, H.-Y.; Jang, S.-H.; Chang, D.-Y.; Kim, W.; Suh-Kim, H.; Lee, J.-H. Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp. Mol. Med. 2014, 46, e110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnishi, S.; Sumiyoshi, H.; Kitamura, S.; Nagaya, N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 2007, 581, 3961–3966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-H.; Jung, J.; Cho, K.J.; Choi, J.-H.; Lee, H.S.; Kim, G.J.; Lee, S.G. Immunomodulatory Effects of Placenta-derived Mesenchymal Stem Cells on T Cells by Regulation of FoxP3 Expression. Int. J. Stem Cells 2018, 11, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Jung, J.; Cho, K.J.; Lee, C.K.; Hwang, S.-G.; Kim, G.J. Comparison of in vitro hepatogenic differentiation potential between various placenta-derived stem cells and other adult stem cells as an alternative source of functional hepatocytes. Differentiation 2012, 84, 223–231. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, K.S.; Jeon, J.H.; Lee, D.R.; Shim, S.H.; Kim, J.K.; Cha, D.-H.; Yoon, T.K.; Kim, G.J. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: A comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011, 346, 53–64. [Google Scholar] [CrossRef]
- Kim, G.D.; Choi, J.H.; Lim, S.M.; Jun, J.H.; Moon, J.W.; Kim, G.J. Alterations in IL-6/STAT3 Signaling by Korean Mistletoe Lectin Regulate the Self-Renewal Activity of Placenta-Derived Mesenchymal Stem Cells. Nutrients 2019, 11, 2604. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Moon, J.W.; Choi, J.-H.; Lee, Y.W.; Park, S.H.; Kim, G.J. Epigenetic Alterations of IL-6/STAT3 Signaling by Placental Stem Cells Promote Hepatic Regeneration in a Rat Model with CCl4-induced Liver Injury. Int. J. Stem Cells 2015, 8, 79–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.; Choi, J.H.; Lee, Y.; Park, J.-W.; Oh, I.-H.; Hwang, S.-G.; Kim, K.-S.; Kim, G.J. Human Placenta-Derived Mesenchymal Stem Cells Promote Hepatic Regeneration in CCl4-Injured Rat Liver Model via Increased Autophagic Mechanism. Stem Cells 2013, 31, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Bin Lee, Y.; Choi, J.H.; Kim, E.N.; Seok, J.; Lee, H.-J.; Yoon, J.H.; Kim, G.J. Human Chorionic Plate-Derived Mesenchymal Stem Cells Restore Hepatic Lipid Metabolism in a Rat Model of Bile Duct Ligation. Stem Cells Int. 2017, 2017, 5180579. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Jun, J.H.; Park, S.Y.; Yang, S.W.; Bae, S.H.; Kim, G.J. Dynamic Regulation of miRNA Expression by Functionally Enhanced Placental Mesenchymal Stem Cells Promotes Hepatic Regeneration in a Rat Model with Bile Duct Ligation. Int. J. Mol. Sci. 2019, 20, 5299. [Google Scholar] [CrossRef] [Green Version]
- Gavins, F.N.E. Are formyl peptide receptors novel targets for therapeutic intervention in ischaemia–reperfusion injury? Trends Pharmacol. Sci. 2010, 31, 266–276. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jang, I.H.; Heo, S.C.; Kim, J.H.; Hwang, N.S. Biomedical therapy using synthetic WKYMVm hexapeptide. Organogenesis 2016, 12, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, I.H.; Heo, S.C.; Kwon, Y.W.; Choi, E.J.; Kim, J.H. Role of formyl peptide receptor 2 in homing of endothelial progenitor cells and therapeutic angiogenesis. Adv. Biol. Regul. 2015, 57, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.C.; Kwon, Y.W.; Jang, I.H.; Jeong, G.O.; Yoon, J.W.; Kim, C.D.; Kwon, S.M.; Bae, Y.-S.; Kim, J.H. WKYMVm-induced activation of formyl peptide receptor 2 stimulates ischemic neovasculogenesis by promoting homing of endothelial colony-forming cells. Stem Cells 2014, 32, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.C.; Kwon, Y.W.; Jang, I.H.; Jeong, G.O.; Lee, T.W.; Yoon, J.W.; Shin, H.J.; Jeong, H.C.; Ahn, Y.; Ko, T.H.; et al. Formyl Peptide Receptor 2 Is Involved in Cardiac Repair After Myocardial Infarction Through Mobilization of Circulating Angiogenic Cells. Stem Cells 2017, 35, 654–665. [Google Scholar] [CrossRef]
- Kwon, Y.W.; Heo, S.C.; Jang, I.H.; Jeong, G.O.; Yoon, J.W.; Mun, J.-H.; Kim, J.H. Stimulation of cutaneous wound healing by an FPR2-specific peptide agonist WKYMVm. Wound Repair Regen. 2015, 23, 575–582. [Google Scholar] [CrossRef]
- Hao, L.; Lei, X.; Zhou, H.; Marshall, A.J.; Liu, L. Critical role for PI3Kγ-dependent neutrophil reactive oxygen species in WKYMVm-induced microvascular hyperpermeability. J. Leukoc. Biol. 2019, 106, 1117–1127. [Google Scholar] [CrossRef]
- Choi, Y.H.; Heo, S.C.; Kwon, Y.W.; Kim, H.D.; Kim, S.H.L.; Jang, I.H.; Kim, J.H.; Hwang, N.S. Injectable PLGA microspheres encapsulating WKYMVM peptide for neovascularization. Acta Biomater. 2015, 25, 76–85. [Google Scholar] [CrossRef]
- Goodwin, A.M.; DAmore, P.A. Wnt signaling in the vasculature. Angiogenesis 2002, 5, 1–9. [Google Scholar] [CrossRef]
- Roeb, E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol. 2018, 68–69, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.H.; Kalinichenko, V.V.; Holterman, A.-X.L.; Wang, X. Transcription factors in liver development, differentiation, and regeneration. Hepatology 2003, 38, 1331–1347. [Google Scholar] [CrossRef]
- Cattaneo, F.; Parisi, M.; Ammendola, R. WKYMVm-induced cross-talk between FPR2 and HGF receptor in human prostate epithelial cell line PNT1A. FEBS Lett. 2013, 587, 1536–1542. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, W.; Yin, L.; Chilian, W.M.; Krieger, J.; Zhang, P. Vascular precursor cells in tissue injury repair. Transl. Res. 2017, 184, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Ober, E.A.; Lemaigre, F.P. Development of the liver: Insights into organ and tissue morphogenesis. J. Hepatol. 2018, 68, 1049–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynaert, H.; Chavez, M.; Geerts, A. Vascular endothelial growth factor and liver regeneration. J. Hepatol. 2001, 34, 759–761. [Google Scholar] [CrossRef]
- Taura, K.; De Minicis, S.; Seki, E.; Hatano, E.; Iwaisako, K.; Osterreicher, C.H.; Kodama, Y.; Miura, K.; Ikai, I.; Uemoto, S.; et al. Hepatic Stellate Cells Secrete Angiopoietin 1 That Induces Angiogenesis in Liver Fibrosis. Gastroenterology 2008, 135, 1729–1738. [Google Scholar] [CrossRef]
- Drixler, T.A.; Vogten, M.J.; Ritchie, E.D.; Van Vroonhoven, T.J.M.V.; Gebbink, M.F.B.G.; Voest, E.E.; Borel Rinkes, I.H.M. Liver Regeneration Is an Angiogenesis- Associated Phenomenon. Ann. Surg. 2002, 236, 703–712. [Google Scholar] [CrossRef]
- Hashimoto, N.; Phan, S.H.; Imaizumi, K.; Matsuo, M.; Nakashima, H.; Kawabe, T.; Shimokata, K.; Hasegawa, Y. Endothelial–Mesenchymal Transition in Bleomycin-Induced Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2010, 43, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Potenta, S.; Zeisberg, E.; Kalluri, R. The role of endothelial-to-mesenchymal transition in cancer progression. Br. J. Cancer 2008, 99, 1375–1379. [Google Scholar] [CrossRef] [Green Version]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Ribera, J.; Pauta, M.; Melgar-Lesmes, P.; Córdoba, B.; Bosch, A.; Calvo, M.; Rodrigo-Torres, D.; Sancho-Bru, P.; Mira, A.; Jiménez, W.; et al. A small population of liver endothelial cells undergoes endothelial-to-mesenchymal transition in response to chronic liver injury. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 313, G492–G504. [Google Scholar] [CrossRef] [PubMed]
- Dufton, N.P.; Peghaire, C.R.; Osuna-Almagro, L.; Raimondi, C.; Kalna, V.; Chauhan, A.; Webb, G.; Yang, Y.; Birdsey, G.M.; Lalor, P.; et al. Dynamic regulation of canonical TGFβ signalling by endothelial transcription factor ERG protects from liver fibrogenesis. Nat. Commun. 2017, 8, 895. [Google Scholar] [CrossRef] [PubMed]
- Park, G.T.; Kwon, Y.W.; Lee, T.W.; Kwon, S.G.; Ko, H.-C.; Kim, M.B.; Kim, J.H. Formyl Peptide Receptor 2 Activation Ameliorates Dermal Fibrosis and Inflammation in Bleomycin-Induced Scleroderma. Front. Immunol. 2019, 10, 2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Li, X.; Chen, Y.; Han, X.; Li, L.; Yang, Z.; Duan, L.; Lu, H.; He, Q. The protective effect of WKYMVm peptide on inflammatory osteolysis through regulating NF-κB and CD9/gp130/STAT3 signalling pathway. J. Cell. Mol. Med. 2020, 24, 1893–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.D.; Kim, Y.-K.; Lee, H.Y.; Kim, Y.-S.; Jeon, S.G.; Baek, S.-H.; Song, D.-K.; Ryu, S.H.; Bae, Y.-S. The Agonists of Formyl Peptide Receptors Prevent Development of Severe Sepsis after Microbial Infection. J. Immunol. 2010, 185, 4302–4310. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-J.; Jung, J.; Na, K.-H.; Moon, J.S.; Lee, H.-J.; Kim, J.-H.; Kim, G.I.; Kwon, S.-W.; Hwang, S.-G.; Kim, G.J. Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl4-injured liver: Potential application to the treatment of hepatic diseases. J. Cell. Biochem. 2010, 111, 1453–1463. [Google Scholar] [CrossRef]
- Jun, J.H.; Choi, J.H.; Bae, S.H.; Oh, S.H.; Kim, G.J. Decreased C-reactive protein induces abnormal vascular structure in a rat model of liver dysfunction induced by bile duct ligation. Clin. Mol. Hepatol. 2016, 22, 372–381. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, J.H.; Park, S.; Kim, J.Y.; Lim, J.-Y.; Park, G.T.; Kim, J.H.; Kim, G.J. Combination Therapy of Placenta-Derived Mesenchymal Stem Cells with WKYMVm Promotes Hepatic Function in a Rat Model with Hepatic Disease via Vascular Remodeling. Cells 2022, 11, 232. https://doi.org/10.3390/cells11020232
Jun JH, Park S, Kim JY, Lim J-Y, Park GT, Kim JH, Kim GJ. Combination Therapy of Placenta-Derived Mesenchymal Stem Cells with WKYMVm Promotes Hepatic Function in a Rat Model with Hepatic Disease via Vascular Remodeling. Cells. 2022; 11(2):232. https://doi.org/10.3390/cells11020232
Chicago/Turabian StyleJun, Ji Hye, Sohae Park, Jae Yeon Kim, Ja-Yun Lim, Gyu Tae Park, Jae Ho Kim, and Gi Jin Kim. 2022. "Combination Therapy of Placenta-Derived Mesenchymal Stem Cells with WKYMVm Promotes Hepatic Function in a Rat Model with Hepatic Disease via Vascular Remodeling" Cells 11, no. 2: 232. https://doi.org/10.3390/cells11020232