Inhibition of PGE2 in Subchondral Bone Attenuates Osteoarthritis
Abstract
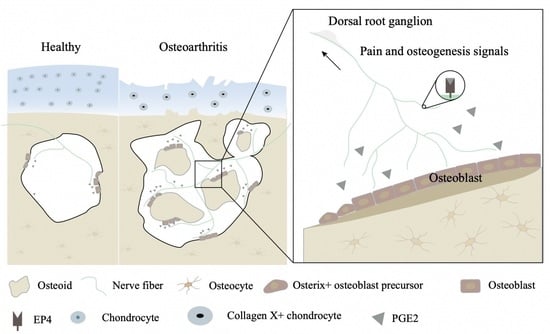
:1. Introduction
2. Methods and Materials
2.1. Mice Preparation and Treatment
2.2. DMM Osteoarthritis Model
2.3. Immunocytochemistry, Immunofluorescence, and Histomorphometry
2.4. μCT Analysis
2.5. ELISA Quantification of PGE2
2.6. Human Sample
2.7. HR-QCT Analysis
2.8. Von Frey Test
2.9. Pressure Threshold
2.10. Voluntary Wheel Running Measurement
2.11. Double Labeling and Histomorphometry Analysis
2.12. Statistics
2.13. Study Approval
3. Results
3.1. Elevated PGE2 in Subchondral Bone Occurs during an Early Stage in DMM Mice
3.2. Elevated PGE2 Is Closely Associated with the Microstructural Alteration of Subchondral Bone in Human Osteoarthritis
3.3. Inhibition of PGE2 Production Attenuates Cartilage Degeneration and Pain
3.4. Inhibition of Excessive PGE2 Production Attenuates the Altered Architecture of Subchondral Bone
3.5. Sensory Denervation Improves Subchondral Bone Architecture and Attenuates Osteoarthritis Progression
3.6. Knockout of EP4 on Sensory Nerve Reduces Osteoarthritis Severity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Gellhorn, A.C.; Katz, J.N.; Suri, P. Osteoarthritis of the spine: The facet joints. Nat. Rev. Rheumatol. 2013, 9, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.J.; Robertsson, O.; Graves, S.; Price, A.J.; Arden, N.K.; Judge, A.; Beard, D.J. Knee replacement. Lancet 2012, 379, 1331–1340. [Google Scholar] [CrossRef]
- Dy, C.J.; Franco, N.; Ma, Y.; Mazumdar, M.; McCarthy, M.M.; Gonzalez Della Valle, A. Complications after patello-femoral versus total knee replacement in the treatment of isolated patello-femoral osteoarthritis. A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012, 20, 2174–2190. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G.; Cook, A.D.; Hamilton, J.A.; Tak, P.P. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol. 2019, 15, 355–363. [Google Scholar] [CrossRef]
- Block, J.A. Osteoarthritis: OA guidelines: Improving care or merely codifying practice? Nat. Rev. Rheumatol. 2014, 10, 324–326. [Google Scholar] [CrossRef]
- Pitsillides, A.A.; Beier, F. Cartilage biology in osteoarthritis--lessons from developmental biology. Nat. Rev. Rheumatol. 2011, 7, 654–663. [Google Scholar] [CrossRef]
- Brandt, K.D.; Radin, E.L.; Dieppe, P.A.; van de Putte, L. Yet more evidence that osteoarthritis is not a cartilage disease. Ann. Rheum Dis. 2006, 65, 1261–1264. [Google Scholar] [CrossRef]
- Sun, Q.; Zhen, G.; Li, T.P.; Guo, Q.; Li, Y.; Su, W.; Xue, P.; Wang, X.; Wan, M.; Guan, Y.; et al. Parathyroid hormone attenuates osteoarthritis pain by remodeling subchondral bone in mice. eLife 2021, 10, e66532. [Google Scholar] [CrossRef]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef]
- Hu, X.; Ji, X.; Yang, M.; Fan, S.; Wang, J.; Lu, M.; Shi, W.; Mei, L.; Xu, C.; Fan, X.; et al. Cdc42 Is Essential for Both Articular Cartilage Degeneration and Subchondral Bone Deterioration in Experimental Osteoarthritis. J. Bone Min. Res. 2018, 33, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B. The importance of subchondral bone in the progression of osteoarthritis. J. Rheumatol Suppl. 2004, 70, 77–80. [Google Scholar] [PubMed]
- Mazur, C.M.; Woo, J.J.; Yee, C.S.; Fields, A.J.; Acevedo, C.; Bailey, K.N.; Kaya, S.; Fowler, T.W.; Lotz, J.C.; Dang, A.; et al. Osteocyte dysfunction promotes osteoarthritis through MMP13-dependent suppression of subchondral bone homeostasis. Bone Res. 2019, 7, 34. [Google Scholar] [CrossRef]
- Zhen, G.; Guo, Q.; Li, Y.; Wu, C.; Zhu, S.; Wang, R.; Guo, X.E.; Kim, B.C.; Huang, J.; Hu, Y.; et al. Mechanical stress determines the configuration of TGFβ activation in articular cartilage. Nat. Commun. 2021, 12, 1706. [Google Scholar] [CrossRef] [PubMed]
- Lories, R.J.; Luyten, F.P. The bone-cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 43–49. [Google Scholar] [CrossRef]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef]
- Bailey, A.J.; Mansell, J.P.; Sims, T.J.; Banse, X. Biochemical and mechanical properties of subchondral bone in osteoarthritis. Biorheology 2004, 41, 349–358. [Google Scholar]
- Mansell, J.P.; Collins, C.; Bailey, A.J. Bone, not cartilage, should be the major focus in osteoarthritis. Nat. Clin. Pr. Rheumatol. 2007, 3, 306–307. [Google Scholar] [CrossRef]
- Mansell, J.P.; Bailey, A.J. Abnormal cancellous bone collagen metabolism in osteoarthritis. J. Clin. Investig. 1998, 101, 1596–1603. [Google Scholar] [CrossRef]
- Su, W.; Liu, G.; Liu, X.; Zhou, Y.; Sun, Q.; Zhen, G.; Wang, X.; Hu, Y.; Gao, P.; Demehri, S.; et al. Angiogenesis stimulated by elevated PDGF-BB in subchondral bone contributes to osteoarthritis development. JCI Insight. 2020, 5, e135446. [Google Scholar] [CrossRef]
- Aaron, R.K.; Racine, J.R.; Voisinet, A.; Evangelista, P.; Dyke, J.P. Subchondral bone circulation in osteoarthritis of the human knee. Osteoarthr. Cartilage. 2018, 26, 940–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Hu, B.; Lv, X.; Zhu, S.; Zhen, G.; Wan, M.; Jain, A.; Gao, B.; Chai, Y.; Yang, M.; et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat. Commun. 2019, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Lv, X.; Chen, H.; Xue, P.; Gao, B.; Wang, X.; Zhen, G.; Crane, J.L.; Pan, D.; Liu, S.; et al. Sensory nerves regulate mesenchymal stromal cell lineage commitment by tuning sympathetic tones. J. Clin. Invest. 2020, 130, 3483–3498. [Google Scholar] [CrossRef] [PubMed]
- Niedermair, T.; Straub, R.H.; Brochhausen, C.; Grässel, S. Impact of the Sensory and Sympathetic Nervous System on Fracture Healing in Ovariectomized Mice. Int. J. Mol. Sci. 2020, 21, 405. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, G.; Liu, D.; Xie, W.; Xiao, W.; Li, Y.; Cai, M. Peripheral nerves in the tibial subchondral bone. Bone Jt. Research. 2022, 11, 439–452. [Google Scholar] [CrossRef]
- Zhu, J.; Zhen, G.; An, S.; Wang, X.; Wan, M.; Li, Y.; Chen, Z.; Guan, Y.; Dong, X.; Hu, Y.; et al. Aberrant subchondral osteoblastic metabolism modifies Na(V)1.8 for osteoarthritis. eLife 2020, 9, e57656. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Southall, M.D.; Vasko, M.R. Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitization of sensory neurons. J. Biol. Chem. 2001, 276, 16083–16091. [Google Scholar] [CrossRef]
- Tu, M.; Yang, M.; Yu, N.; Zhen, G.; Wan, M.; Liu, W.; Ji, B.; Ma, H.; Guo, Q.; Tong, P.; et al. Inhibition of cyclooxygenase-2 activity in subchondral bone modifies a subtype of osteoarthritis. Bone Res. 2019, 7, 29. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, Y.; Zhang, S.; Ding, Y.; Huo, K.; Yang, J.; Zhao, L.; Nian, B.; Zhong, T.P.; Lu, W.; et al. PGE2 activates EP4 in subchondral bone osteoclasts to regulate osteoarthritis. Bone Res. 2022, 10, 27. [Google Scholar] [CrossRef]
- Fukai, A.; Kamekura, S.; Chikazu, D.; Nakagawa, T.; Hirata, M.; Saito, T.; Hosaka, Y.; Ikeda, T.; Nakamura, K.; Chung, U.I.; et al. Lack of a chondroprotective effect of cyclooxygenase 2 inhibition in a surgically induced model of osteoarthritis in mice. Arthritis Rheum. 2012, 64, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, B.; Liu, C.J. Establishment of a surgically-induced model in mice to investigate the protective role of progranulin in osteoarthritis. J. Vis. Exp. 2014, e50924. [Google Scholar] [CrossRef] [PubMed]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartilage. 2006, 14, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, G.R.; Bourne, R.B.; Noble, P.C.; Benjamin, J.B.; Lonner, J.H.; Scott, W.N. The new Knee Society Knee Scoring System. Clin. Orthop Relat Res. 2012, 470, 3–19. [Google Scholar] [CrossRef]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar]
- Haversath, M.; Catelas, I.; Li, X.; Tassemeier, T.; Jäger, M. PGE₂ and BMP-2 in bone and cartilage metabolism: 2 intertwining pathways. Can. J. Physiol Pharmacol. 2012, 90, 1434–1445. [Google Scholar] [CrossRef]
- Ni, S.; Ling, Z.; Wang, X.; Cao, Y.; Wu, T.; Deng, R.; Crane, J.L.; Skolasky, R.; Demehri, S.; Zhen, G.; et al. Sensory innervation in porous endplates by Netrin-1 from osteoclasts mediates PGE2-induced spinal hypersensitivity in mice. Nat. Commun. 2019, 10, 5643. [Google Scholar] [CrossRef]
- Sakuma, Y.; Li, Z.; Pilbeam, C.C.; Alander, C.B.; Chikazu, D.; Kawaguchi, H.; Raisz, L.G. Stimulation of cAMP production and cyclooxygenase-2 by prostaglandin E(2) and selective prostaglandin receptor agonists in murine osteoblastic cells. Bone 2004, 34, 827–834. [Google Scholar] [CrossRef]
- Krasselt, M.; Baerwald, C. Celecoxib for the treatment of musculoskeletal arthritis. Expert Opin. Pharmacother. 2019, 20, 1689–1702. [Google Scholar] [CrossRef]
- Huang, W.N.; Tso, T.K. Etoricoxib improves osteoarthritis pain relief, joint function, and quality of life in the extreme elderly. Bosn J. Basic Med. Sci. 2018, 18, 87–94. [Google Scholar] [CrossRef]
- Bannwarth, B.; Bérenbaum, F. Lumiracoxib in the management of osteoarthritis and acute pain. Expert Opin Pharmacother. 2007, 8, 1551–1564. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wu, D.; Sang, L.; Wang, Y.; Shen, Y.; Zhuang, X.; Chu, M.; Jiang, L. Comparative effectiveness of glucosamine, chondroitin, acetaminophen or celecoxib for the treatment of knee and/or hip osteoarthritis: A network meta-analysis. Clin. Exp. Rheumatol. 2018, 36, 595–602. [Google Scholar] [PubMed]
- Puljak, L.; Marin, A.; Vrdoljak, D.; Markotic, F.; Utrobicic, A.; Tugwell, P. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017, 5, Cd009865. [Google Scholar] [PubMed]
- Aoyama, T.; Liang, B.; Okamoto, T.; Matsusaki, T.; Nishijo, K.; Ishibe, T.; Yasura, K.; Nagayama, S.; Nakayama, T.; Nakamura, T.; et al. PGE2 signal through EP2 promotes the growth of articular chondrocytes. J. Bone Min. Res. 2005, 20, 377–389. [Google Scholar]
- Attur, M.; Al-Mussawir, H.E.; Patel, J.; Kitay, A.; Dave, M.; Palmer, G.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 exerts catabolic effects in osteoarthritis cartilage: Evidence for signaling via the EP4 receptor. J. Immunol. 2008, 181, 5082–5088. [Google Scholar] [CrossRef] [PubMed]
- Timur, U.T.; Caron, M.M.J.; Jeuken, R.M.; Bastiaansen-Jenniskens, Y.M.; Welting, T.J.M.; van Rhijn, L.W.; van Osch, G.; Emans, P.J. Chondroprotective Actions of Selective COX-2 Inhibitors In Vivo: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 6962. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, J.; Zhen, G.; Hu, Y.; An, S.; Li, Y.; Zheng, Q.; Chen, Z.; Yang, Y.; Wan, M.; et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Investig. 2019, 129, 1076–1093. [Google Scholar]
- Liu, S.; Wang, Q.; Li, Z.; Ma, L.; Li, T.; Li, Y.; Wang, N.; Liu, C.; Xue, P.; Wang, C. TRPV1 Channel Activated by the PGE2/EP4 Pathway Mediates Spinal Hypersensitivity in a Mouse Model of Vertebral Endplate Degeneration. Oxid Med. Cell Longev. 2021, 2021, 9965737. [Google Scholar]
- Scala, R.; Maqoud, F.; Angelelli, M.; Latorre, R.; Perrone, M.G.; Scilimati, A.; Tricarico, D. Zoledronic Acid Modulation of TRPV1 Channel Currents in Osteoblast Cell Line and Native Rat and Mouse Bone Marrow-Derived Osteoblasts: Cell Proliferation and Mineralization Effect. Cancers 2019, 11, 206. [Google Scholar]
- da Costa, F.L.P.; Pinto, M.C.X.; Santos, D.C.; Carobin, N.V.; de Jesus, I.C.G.; Ferreira, L.A.; Guatimosim, S.; Silva, J.F.; Castro Junior, C.J. Ketamine potentiates TRPV1 receptor signaling in the peripheral nociceptive pathways. Biochem. Pharmacol. 2020, 182, 114210. [Google Scholar]
- Veilleux-Lemieux, D.; Castel, A.; Carrier, D.; Beaudry, F.; Vachon, P. Pharmacokinetics of ketamine and xylazine in young and old Sprague-Dawley rats. J. Am. Assoc. Lab. Anim Sci. 2013, 52, 567–570. [Google Scholar] [PubMed]
- Curtis, E.; Fuggle, N.; Shaw, S.; Spooner, L.; Ntani, G.; Parsons, C.; Corp, N.; Honvo, G.; Baird, J.; Maggi, S.; et al. Safety of Cyclooxygenase-2 Inhibitors in Osteoarthritis: Outcomes of a Systematic Review and Meta-Analysis. Drugs Aging. 2019, 36, 25–44. [Google Scholar] [PubMed]
- Massicotte, F.; Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P.; Lajeunesse, D. Modulation of insulin-like growth factor 1 levels in human osteoarthritic subchondral bone osteoblasts. Bone 2006, 38, 333–341. [Google Scholar] [PubMed]
- Nishitani, K.; Ito, H.; Hiramitsu, T.; Tsutsumi, R.; Tanida, S.; Kitaori, T.; Yoshitomi, H.; Kobayashi, M.; Nakamura, T. PGE2 inhibits MMP expression by suppressing MKK4-JNK MAP kinase-c-JUN pathway via EP4 in human articular chondrocytes. J. Cell Biochem. 2010, 109, 425–433. [Google Scholar]
- Jin, Y.; Liu, Q.; Chen, P.; Zhao, S.; Jiang, W.; Wang, F.; Li, P.; Zhang, Y.; Lu, W.; Zhong, T.P.; et al. A novel prostaglandin E receptor 4 (EP4) small molecule antagonist induces articular cartilage regeneration. Cell Discov. 2022, 8, 24. [Google Scholar]
- Takita, M.; Inada, M.; Maruyama, T.; Miyaura, C. Prostaglandin E receptor EP4 antagonist suppresses osteolysis due to bone metastasis of mouse malignant melanoma cells. FEBS Letters 2007, 581, 565–571. [Google Scholar] [CrossRef]
- Na, H.-K.; Park, J.-M.; Lee, H.G.; Lee, H.N.; Myung, S.-J.; Surh, Y.-J. 15-Hydroxyprostaglandin dehydrogenase as a novel molecular target for cancer chemoprevention and therapy. Biochem. Pharmacol. 2011, 82, 1352–1360. [Google Scholar]
- Geiger, B.C.; Wang, S.; Padera, R.F., Jr.; Grodzinsky, A.J.; Hammond, P.T. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 2018, 10, eaat8800. [Google Scholar]
- Deng, Q.; Li, P.; Che, M.; Liu, J.; Biswas, S.; Ma, G.; He, L.; Wei, Z.; Zhang, Z.; Yang, Y.; et al. Activation of hedgehog signaling in mesenchymal stem cells induces cartilage and bone tumor formation via Wnt/β-Catenin. eLife 2019, 8, e50208. [Google Scholar]
- Weinstock, A.; Rahman, K.; Yaacov, O.; Nishi, H.; Menon, P.; Nikain, C.A.; Garabedian, M.L.; Pena, S.; Akbar, N.; Sansbury, B.E.; et al. Wnt signaling enhances macrophage responses to IL-4 and promotes resolution of atherosclerosis. eLife 2021, 10, e67932. [Google Scholar]
- Hartley, A.; Sanderson, E.; Paternoster, L.; Teumer, A.; Kaplan, R.C.; Tobias, J.H.; Gregson, C.L. Mendelian randomization provides evidence for a causal effect of higher serum IGF-1 concentration on risk of hip and knee osteoarthritis. Rheumatology 2021, 60, 1676–1686. [Google Scholar] [PubMed]
- McCarthy, T.L.; Centrella, M. Androgen receptor activation integrates complex transcriptional effects in osteoblasts, involving the growth factors TGF-β and IGF-I, and transcription factor C/EBPδ. Gene 2015, 573, 129–140. [Google Scholar] [PubMed]
- McCarthy, T.L.; Yun, Z.; Madri, J.A.; Centrella, M. Stratified control of IGF-I expression by hypoxia and stress hormones in osteoblasts. Gene 2014, 539, 141–151. [Google Scholar] [PubMed] [Green Version]
- Hilal, G.; Massicotte, F.; Martel-Pelletier, J.; Fernandes, J.C.; Pelletier, J.-P.; Lajeunesse, D. Endogenous Prostaglandin E2 and Insulin-like Growth Factor 1 Can Modulate the Levels of Parathyroid Hormone Receptor in Human Osteoarthritic Osteoblasts. J. Bone Miner. Res. 2001, 16, 713–721. [Google Scholar]
- Davis, F.M.; Tsoi, L.C.; Wasikowski, R.; denDekker, A.; Joshi, A.; Wilke, C.; Deng, H.; Wolf, S.; Obi, A.; Huang, S.; et al. Epigenetic regulation of the PGE2 pathway modulates macrophage phenotype in normal and pathologic wound repair. JCI Insight. 2020, 5, e138443. [Google Scholar]






| Reagent Type | Designation | Source | Identifiers |
|---|---|---|---|
| primary antibody | calcitonin gene-related peptide (CGRP) | ab81887 | Abcam |
| MMP13 | ab39012 | Abcam | |
| osterix | ab22552 | Abcam | |
| COLX | ab58632 | Abcam | |
| osteocalcin | M188 | Takara | |
| COX-2 | ab15191 | Abcam | |
| secondary antibody | goat anti-rat IgG (Alexa Fluor® 594) | ab150160 | Abcam |
| goat anti-mouse IgG (Alexa Fluor® 488) | ab150113 | Abcam | |
| goat anti-rabbit IgG (Alexa Fluor® 488) | ab150077 | Abcam | |
| goat anti-rabbit IgG (HRP) | ab205718 | Abcam |
| Normal | Osteoarthritis | |
|---|---|---|
| Sample size | 8 | 8 |
| Gender (M/F) | 4/4 | 4/4 |
| Height (cm) | 174.3 ± 4.1 | 170.1 ± 3.9 |
| Body weight (kg) | 67.8 ± 5.9 | 72.1 ± 4.9 |
| Age (y) | 36.5 ± 4.7 | 62.3 + 2.5 |
| BMI | 23.6 ± 2.6 | 25.2 ± 3.1 |
| Varus deformity (°) | N/A | 11.4 ± 2.6 |
| Knee society score [34] | N/A | 54.2 ± 5.7 |
| Classification | Detail |
|---|---|
| Non-lesion area | intact cartilage |
| Moderate area | minimal or overt fibrillation of cartilage |
| Lesion area | partial cartilage erosion, and exposures of subchondral bone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Zhang, Y.; Ding, Y.; Xie, W.; Li, H.; Li, S.; Li, Y.; Cai, M. Inhibition of PGE2 in Subchondral Bone Attenuates Osteoarthritis. Cells 2022, 11, 2760. https://doi.org/10.3390/cells11172760
Sun Q, Zhang Y, Ding Y, Xie W, Li H, Li S, Li Y, Cai M. Inhibition of PGE2 in Subchondral Bone Attenuates Osteoarthritis. Cells. 2022; 11(17):2760. https://doi.org/10.3390/cells11172760
Chicago/Turabian StyleSun, Qi, Yuanzhen Zhang, Yilan Ding, Wenqing Xie, Hengzhen Li, Shaohua Li, Yusheng Li, and Ming Cai. 2022. "Inhibition of PGE2 in Subchondral Bone Attenuates Osteoarthritis" Cells 11, no. 17: 2760. https://doi.org/10.3390/cells11172760