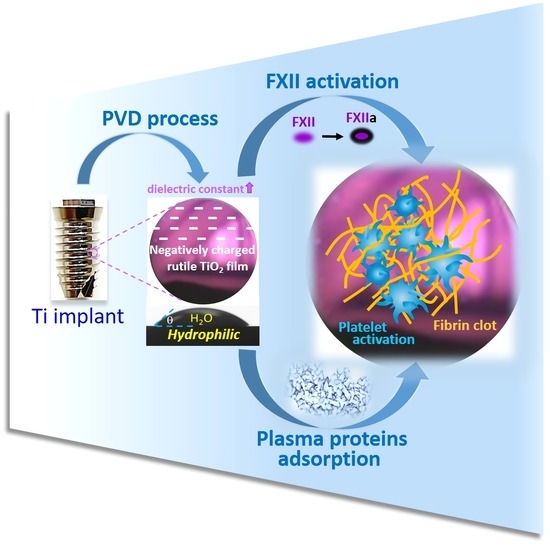
Blood Coagulation on Titanium Dioxide Films with Various Crystal Structures on Titanium Implant Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of TiO2 Film Specimens
2.2. Surface Characterizations
2.3. Clotting Kinetics of Whole Blood
2.4. Factor XII Activation
2.5. Fibrinogen Adsorption
2.6. Fibrin Attachment
2.7. Platelet Adhesion
2.8. Statistical Analysis
3. Results and Discussion
3.1. Surface Characterization
3.1.1. Surface Topography
3.1.2. TiO2 Film Thickness
3.1.3. TiO2 Crystal Phase Structure
3.1.4. Surface Energy and Dielectric Constant
3.2. Clotting Kinetics of Whole Blood
3.3. Factor XII (FXII) Activation
3.4. Fibrinogen Adsorption
3.5. Fibrin Attachment
3.6. Platelet Adhesion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayne, S.C. Dental Biomaterials: Where Are We and Where Are We Going? J. Dent. Educ. 2005, 69, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where We Have Been and Where We Are Going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.B.; Rodriguez, I.; Venkatraman, S.S. The effect of topography of polymer surfaces on platelet adhesion. Biomaterials 2010, 31, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Davies, J.E. Red blood cell and platelet interactions with titanium implant surfaces. Clin. Oral Implant. Res. 2000, 11, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Abdulmajeed, A.A.; Walboomers, X.F.; Massera, J.; Kokkari, A.K.; Vallittu, P.K.; Närhi, T.O. Blood and fibroblast responses to thermoset BisGMA-TEGDMA/glass fiber-reinforced composite implants in vitro. Clin. Oral Implant. Res. 2013, 25, 843–851. [Google Scholar] [CrossRef]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.; Xiao, Y. Formation of Blood Clot on Biomaterial Implants Influences Bone Healing. Tissue Eng. Part B Rev. 2014, 20, 697–712. [Google Scholar] [CrossRef]
- Vera, M.L.; Schuster, J.; Rosenberger, M.R.; Bernard, H.; Schvezov, C.E.; Ares, A.E. Evaluation of the Haemocompatibility of TiO2 Coatings Obtained by Anodic Oxidation of Ti-6Al-4V. Procedia Mater. Sci. 2015, 8, 366–374. [Google Scholar] [CrossRef]
- Wells, L.A.; Guo, H.; Emili, A.; Sefton, M.V. The profile of adsorbed plasma and serum proteins on methacrylic acid copolymer beads: Effect on complement activation. Biomaterials 2017, 118, 74–83. [Google Scholar] [CrossRef]
- Ekdahl, K.N.; Davoodpour, P.; Ekstrand-Hammarström, B.; Fromell, K.; Hamad, O.A.; Hong, J.; Bucht, A.; Mohlin, C.; Seisenbaeva, G.A.; Kessler, V.; et al. Contact (kallikrein/kinin) system activation in whole human blood induced by low concentrations of α-Fe2O3 nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 735–744. [Google Scholar] [CrossRef]
- Jaffer, I.H.; Fredenburgh, J.C.; Hirsh, J.; Weitz, J.I. Medical device-induced thrombosis: What causes it and how can we prevent it? J. Thromb. Haemost. 2015, 13, S72–S81. [Google Scholar] [CrossRef] [PubMed]
- Sabino, R.M.; Kauk, K.; Movafaghi, S.; Kota, A.; Popat, K.C. Interaction of blood plasma proteins with superhemophobic titania nanotube surfaces. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102046. [Google Scholar] [CrossRef] [PubMed]
- de Mel, A.; Cousins, B.G.; Seifalian, A. Surface Modification of Biomaterials: A Quest for Blood Compatibility. Int. J. Biomater. 2012, 2012, 707863. [Google Scholar] [CrossRef] [PubMed]
- Manivasagam, V.K.; Popat, K.C. In Vitro Investigation of Hemocompatibility of Hydrothermally Treated Titanium and Titanium Alloy Surfaces. ACS Omega 2020, 5, 8108–8120. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, C.A. Hemocompatibility of Biomaterials for Clinical Applications: Blood-Biomaterials Interactions; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Yeo, I.-S.L. Modifications of Dental Implant Surfaces at the Micro- and Nano-Level for Enhanced Osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Yoshinari, M.; Matsuzaka, K.; Inoue, T. Surface modification by cold-plasma technique for dental implants—Bio-functionalization with binding pharmaceuticals. Jpn. Dent. Sci. Rev. 2011, 47, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. Advanced titanium dioxide-polytetrafluorethylene (TiO2-PTFE) nanocomposite coatings on stainless steel surfaces with antibacterial and anti-corrosion properties. Appl. Surf. Sci. 2019, 490, 231–241. [Google Scholar] [CrossRef]
- Shim, J.W.; Bae, I.-H.; Jeong, M.H.; Park, D.S.; Lim, K.-S.; Kim, J.U.; Kim, M.-K.; Kim, J.H.; Sim, D.S. Effects of a Titanium Dioxide Thin Film for Improving the Biocompatibility of Diamond-Like Coated Coronary Stents. Met. Mater. Int. 2019, 26, 1455–1462. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Fromell, K.; Vinogradov, V.V.; Terekhov, A.N.; Pakhomov, A.V.; Nilsson, E.K.; Ekdahl, K.N.; Vinogradov, V.; Kessler, V.G. Dispersion of TiO2 nanoparticles improves burn wound healing and tissue regeneration through specific interaction with blood serum proteins. Sci. Rep. 2017, 7, 15448. [Google Scholar] [CrossRef] [Green Version]
- Fathi-Hafshejani, P.; Johnson, H.; Ahmadi, Z.; Roach, M.; Shamsaei, N.; Mahjouri-Samani, M. Phase-Selective and Localized TiO2 Coating on Additive and Wrought Titanium by a Direct Laser Surface Modification Approach. ACS Omega 2020, 5, 16744–16751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, G.; Zhang, X.; Liu, Z.; Guo, Z.; Ullah, I.; Zhang, S.; Man, J.; Li, D. Systematically evaluate the physicochemical property and hemocompatibility of phase dependent TiO2 on medical pure titanium. Surf. Coatings Technol. 2020, 404, 126501. [Google Scholar] [CrossRef]
- Benčina, M.; Iglič, A.; Mozetič, M.; Junkar, I. Crystallized TiO2 Nanosurfaces in Biomedical Applications. Nanomaterials 2020, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, J.; Lv, K.; Zhang, W.; Ding, X.; Yang, G.; Liu, X.; Jiang, X. Surface thermal oxidation on titanium implants to enhance osteogenic activity and in vivo osseointegration. Sci. Rep. 2016, 6, 31769. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhang, K.; Wu, C.; Lei, X.; Ding, J.; Shi, X.; Liu, C. Micro-Arc Oxidation Enhances the Blood Compatibility of Ultrafine-Grained Pure Titanium. Materials 2017, 10, 1446. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-T.; Lin, H.-I.; Chung, C.-J.; Tang, C.-H.; He, J.-L. Osseointegrating and phase-oriented micro-arc-oxidized titanium dioxide bone implants. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211006878. [Google Scholar] [CrossRef]
- López-Huerta, F.; Cervantes, B.; González, O.; Hernández-Torres, J.; García-González, L.; Vega, R.; Herrera-May, A.L.; Soto, E. Biocompatibility and Surface Properties of TiO2 Thin Films Deposited by DC Magnetron Sputtering. Materials 2014, 7, 4105–4117. [Google Scholar] [CrossRef] [Green Version]
- Vera, M.L.; Rosenberger, M.R.; Schvezov, C.E.; Ares, A.E. Fabrication of TiO2 Crystalline Coatings by Combining Ti-6Al-4V Anodic Oxidation and Heat Treatments. Int. J. Biomater. 2015, 2015, 395657. [Google Scholar] [CrossRef] [Green Version]
- Amarnath, L.P.; Srinivas, A.; Ramamurthi, A. In vitro hemocompatibility testing of UV-modified hyaluronan hydrogels. Biomaterials 2006, 27, 1416–1424. [Google Scholar] [CrossRef]
- Major, S.; Cyrus, P.; Hubálovská, M. The Influence of Surface Roughness on Biocompatibility and Fatigue Life of Titanium Based Alloys. IOP Conf. Ser. Mater. Sci. Eng. 2017, 175, 12053. [Google Scholar] [CrossRef] [Green Version]
- Tsunoda, N.; Kokubo, K.-I.; Sakai, K.; Fukuda, M.; Miyazaki, M.; Hiyoshi, T. Surface Roughness of Cellulose Hollow Fiber Dialysis Membranes and Platelet Adhesion. ASAIO J. 1999, 45, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, T.; Ishimaru, T.; Kamijo, A.; Yoshimoto, Y.; Yoshimura, T.; Yohena, S.; Kodama, H.; Hotta, A.; Takahashi, K.; Suzuki, T. Effects of surface roughness on anti-thrombogenicity of diamond-like carbon films. Diam. Relat. Mater. 2007, 16, 1343–1348. [Google Scholar] [CrossRef]
- Chen, J.; Yang, P.; Liao, Y.; Wang, J.; Chen, H.; Sun, H.; Huang, N. Effect of the Duration of UV Irradiation on the Anticoagulant Properties of Titanium Dioxide Films. ACS Appl. Mater. Interfaces 2015, 7, 4423–4432. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, M.; Pellicer, E.; Sort, J.; Baró, M.D.; Kovač, J.; Novak, S.; Kobe, S. Improvement to the Corrosion Resistance of Ti-Based Implants Using Hydrothermally Synthesized Nanostructured Anatase Coatings. Materials 2014, 7, 180–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, N.S.; Mahdjoub, N.; Vishnyakov, V.; Kelly, P.J.; Kriek, R.J. The effect of crystalline phase (anatase, brookite and rutile) and size on the photocatalytic activity of calcined polymorphic titanium dioxide (TiO2). Polym. Degrad. Stab. 2018, 150, 31–36. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, Y.; Zheng, D.; Song, R.; Zhang, Y.; Jiang, P.; Vogler, E.A.; Lin, C. Effect of construction of TiO2 nanotubes on platelet behaviors: Structure-property relationships. Acta Biomater. 2017, 51, 505–512. [Google Scholar] [CrossRef]
- Lv, L.; Li, K.; Xie, Y.; Cao, Y.; Zheng, X. Enhanced osteogenic activity of anatase TiO2 film: Surface hydroxyl groups induce conformational changes in fibronectin. Mater. Sci. Eng. C 2017, 78, 96–104. [Google Scholar] [CrossRef]
- Movafaghi, S.; Wang, W.; Bark, D.L.; Dasi, L.P.; Popat, K.C.; Kota, A.K. Hemocompatibility of super-repellent surfaces: Current and future. Mater. Horizons 2019, 6, 1596–1610. [Google Scholar] [CrossRef]
- Hong, J.; Kurt, S.; Thor, A. A Hydrophilic Dental Implant Surface Exhibit Thrombogenic Properties In Vitro. Clin. Implant Dent. Relat. Res. 2013, 15, 105–112. [Google Scholar] [CrossRef]
- Xu, L.-C.; Bauer, J.W.; Siedlecki, C.A. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef] [Green Version]
- Eder, D.; Kinloch, I.A.; Windle, A.H. Pure rutile nanotubes. Chem. Commun. 2006, 1448–1450. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jung, H.S.; Noh, J.H.; Kim, J.-R.; Hong, K.S. Influence of anatase-rutile phase transformation on dielectric properties of sol-gel derived TiO2 thin films. J. Electroceramics 2006, 16, 447–451. [Google Scholar] [CrossRef]
- Shiau, D.-K.; Yang, C.-H.; Sun, Y.-S.; Wu, M.-F.; Pan, H.; Huang, H.-H. Enhancing the blood response and antibacterial adhesion of titanium surface through oxygen plasma immersion ion implantation treatment. Surf. Coatings Technol. 2019, 365, 173–178. [Google Scholar] [CrossRef]
- Oshida, Y. Oxidation and oxides. In Bioscience and Bioengineering of Titanium Materials; Yoshida, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 79–103. [Google Scholar]
- Arora, C.D.H.; Yuan, Y.; Boyle, J.; Petras, K.; Rabatic, B.; Paunesku, T.; Woloschak, G. Titanium dioxide nanocomposites. In Nanotechnologies for the Life Sciences; Kumar, C.S.S.R., Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 28–36. [Google Scholar]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liao, X.; Fok, A.; Ning, C.; Ng, P.; Wang, Y. Effect of crystalline phase changes in titania (TiO2) nanotube coatings on platelet adhesion and activation. Mater. Sci. Eng. C 2018, 82, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Simon-Walker, R.; Cavicchia, J.; Prawel, D.A.; Dasi, L.P.; James, S.P.; Popat, K.C. Hemocompatibility of hyaluronan enhanced linear low density polyethylene for blood contacting applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1964–1975. [Google Scholar] [CrossRef]
- Maitz, M.F.; Pham, M.-T.; Wieser, E.; Tsyganov, I. Blood Compatibility of Titanium Oxides with Various Crystal Structure and Element Doping. J. Biomater. Appl. 2003, 17, 303–319. [Google Scholar] [CrossRef]
- Zhuo, R.; Siedlecki, C.A.; Vogler, E.A. Autoactivation of blood factor XII at hydrophilic and hydrophobic surfaces. Biomaterials 2006, 27, 4325–4332. [Google Scholar] [CrossRef]
- Bauer, J.W.; Xu, L.-C.; Vogler, E.A.; Siedlecki, C.A. Surface dependent contact activation of factor XII and blood plasma coagulation induced by mixed thiol surfaces. Biointerphases 2017, 12, 02D410. [Google Scholar] [CrossRef] [Green Version]
- Basmadjian, D.; Sefton, M.V.; Baldwin, S.A. Coagulation on biomaterials in flowing blood: Some theoretical considerations. Biomaterials 1997, 18, 1511–1522. [Google Scholar] [CrossRef]
- Brebels, J.; Manca, J.V.; Lutsen, L.; Vanderzande, D.; Maes, W. High dielectric constant conjugated materials for organic photovoltaics. J. Mater. Chem. A 2017, 5, 24037–24050. [Google Scholar] [CrossRef]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Szułdrzyński, K.; Jankowski, M.; Potaczek, D.P.; Undas, A. Plasma Fibrin Clot Properties as Determinants of Bleeding Time in Human Subjects: Association with Histidine-Rich Glycoprotein. Dis. Markers 2020, 2020, 7190828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurens, N.; Koolwijk, P.; De Maat, M.P.M. Fibrin structure and wound healing. J. Thromb. Haemost. 2006, 4, 932–939. [Google Scholar] [CrossRef]
- Modery-Pawlowski, C.L.; Tian, L.L.; Pan, V.; McCrae, K.R.; Mitragotri, S.; Gupta, A.S. Approaches to synthetic platelet analogs. Biomaterials 2013, 34, 526–541. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef] [Green Version]
- Cines, D.; Lebedeva, T.; Nagaswami, C.; Hayes, V.; Massefski, W.; Litvinov, R.; Rauova, L.; Lowery, T.J.; Weisel, J.W. Clot contraction: Compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood 2014, 123, 1596–1603. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.V.; Litvinov, R.I.; Alber, M.S.; Weisel, J.W. Quantitative structural mechanobiology of platelet-driven blood clot contraction. Nat. Commun. 2017, 8, 1274. [Google Scholar] [CrossRef]
- Nandi, S.; Sproul, E.P.; Nellenbach, K.; Erb, M.; Gaffney, L.; Freytes, D.O.; Brown, A.C. Platelet-like particles dynamically stiffen fibrin matrices and improve wound healing outcomes. Biomater. Sci. 2019, 7, 669–682. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Mechanisms of fibrin polymerization and clinical implications. Blood 2013, 121, 1712–1719. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, S.N.; Gonçalves, I.C.; Martins, M.; Barbosa, M.A.; Ratner, B.D. Fibrinogen adsorption, platelet adhesion and activation on mixed hydroxyl-/methyl-terminated self-assembled monolayers. Biomaterials 2006, 27, 5357–5367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, Y.; Hauch, K.; Horbett, T.A. Fibrinogen and von Willebrand factor mediated platelet adhesion to polystyrene under flow conditions. J. Biomater. Sci. Polym. Ed. 2008, 19, 1383–1410. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Prado, R.; Orive, G.; Tejero, R. Effects of calcium-modified titanium implant surfaces on platelet activation, clot formation, and osseointegration. J. Biomed. Mater. Res. Part A 2015, 103, 969–980. [Google Scholar] [CrossRef] [PubMed]








| Contact Angle (θ) | Surface Energy (m/Nm) | Dielectric Constant | ||
|---|---|---|---|---|
| di-H2O | Glycerol | (ɛ) | ||
| T | 44.59 ± 1.77 | 54.62 ± 4.91 | 752.68 | 81.9 |
| A | 44.01 ± 0.46 | 52.55 ± 2.80 | 756.43 | 24.9 |
| AR | 47.44 ± 0.88 | 68.28 ± 1.76 | 760.86 | 39.6 |
| R | 53.95 ± 2.26 | 66.50 ± 1.78 | 759.69 | 143.7 |
| G | 32.15 ± 1.41 | 50.19 ± 2.46 | 750.03 | 3.8 * |
| * Reference from https://www.clippercontrols.com/pages/DielectricConstant-Values.html#G (accessed on 16 August 2022) | ||||
| Atomic Percentage (at.%) | ||||
|---|---|---|---|---|
| Ti | O | C | N | |
| T | 33.1 | 58.9 | 7.3 | 0.8 |
| A | 34.6 | 56.0 | 8.8 | 0.6 |
| AR | 35.0 | 55.4 | 9.1 | 0.6 |
| R | 33.7 | 52.6 | 12.6 | 1.1 |
| G | - | - | 86.4 | 13.6 * |
| Atomic Percentage (at.%) | ||||
|---|---|---|---|---|
| Ti | O | C | N | |
| T | 38.9 | 58.5 | 1.7 | 0.9 |
| A | 34.3 | 55.1 | 9.1 | 1.5 |
| AR | 36.7 | 56.5 | 5.6 | 1.2 |
| R | 34.4 | 52.9 | 11.4 | 1.3 |
| G | - | - | 78.4 | 21.6 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-H.; Chen, Z.-H.; Nguyen, D.T.; Tseng, C.-M.; Chen, C.-S.; Chang, J.-H. Blood Coagulation on Titanium Dioxide Films with Various Crystal Structures on Titanium Implant Surfaces. Cells 2022, 11, 2623. https://doi.org/10.3390/cells11172623
Huang H-H, Chen Z-H, Nguyen DT, Tseng C-M, Chen C-S, Chang J-H. Blood Coagulation on Titanium Dioxide Films with Various Crystal Structures on Titanium Implant Surfaces. Cells. 2022; 11(17):2623. https://doi.org/10.3390/cells11172623
Chicago/Turabian StyleHuang, Her-Hsiung, Zhi-Hwa Chen, Diem Thuy Nguyen, Chuan-Ming Tseng, Chiang-Sang Chen, and Jean-Heng Chang. 2022. "Blood Coagulation on Titanium Dioxide Films with Various Crystal Structures on Titanium Implant Surfaces" Cells 11, no. 17: 2623. https://doi.org/10.3390/cells11172623