Targeting on Nrf2/Sesn2 Signaling to Rescue Cardiac Dysfunction during High-Fat Diet-Induced Obesity
Abstract
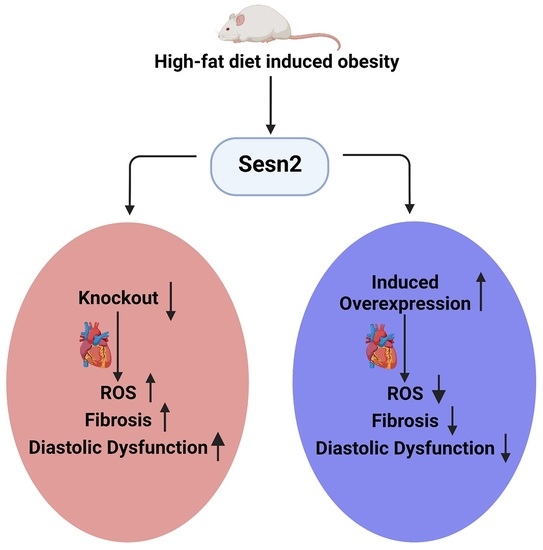
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Human Tissue Samples
2.3. Echocardiographic Evaluation
2.4. Histopathology
2.5. Western Blot Analysis
2.6. Measurement of Mitochondrial Reactive Oxygen Species
2.7. Statistical Analysis
2.8. Data and Resource Availability
3. Results
3.1. Weight Gain Variation in Cardiomyocyte Sesn2 Knockout and Overexpressed Mice
3.2. Relationship between Sesn2 and Nrf2 in Cardiomyocytes
3.3. Sesn2 Preserves Cardiac Function in Obese Mice
3.4. The Overexpression of Sesn2 Provided Cardiac Protection from Fibrosis
3.5. ROS Presence in Obese Mice Cardiac Tissue
3.6. Effects of Obesity on Human Cardiac Tissue
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badimon, L.; Bugiardini, R.; Cenko, E.; Cubedo, J.; Dorobantu, M.; Duncker, D.J.; Estruch, R.; Milicic, D.; Tousoulis, D.; Vasiljevic, Z.; et al. Position Paper of the European Society of Cardiology–Working Group of Coronary Pathophysiology and Microcirculation: Obesity and Heart Disease. Eur. Heart J. 2017, 38, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.; Adam, S.; Ho, J.H.; Iqbal, Z.; Turkington, P.; Razvi, S.; Le Roux, C.W.; Soran, H.; Syed, A.A. Obesity: A Critical Risk Factor in the COVID-19 Pandemic. Clin. Obes. 2020, 10, e12403. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Magkos, F. The Epidemiology of Obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and Inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef]
- Tarantini, S.; Valcarcel-Ares, M.N.; Yabluchanskiy, A.; Tucsek, Z.; Hertelendy, P.; Kiss, T.; Gautam, T.; Zhang, X.A.; Sonntag, W.E.; de Cabo, R.; et al. Nrf2 Deficiency Exacerbates Obesity-Induced Oxidative Stress, Neurovascular Dysfunction, Blood–Brain Barrier Disruption, Neuroinflammation, Amyloidogenic Gene Expression, and Cognitive Decline in Mice, Mimicking the Aging Phenotype. J. Gerontol. Ser. A 2018, 73, 853–863. [Google Scholar] [CrossRef]
- Jimoh, A.; Tanko, Y.; Ahmed, A.; Mohammed, A.; Ayo, J.O. Resveratrol Prevents High-Fat Diet-Induced Obesity and Oxidative Stress in Rabbits. Pathophysiology 2018, 25, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative Stress and Inflammation Interactions in Human Obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Innes, K.E.; Vincent, K.R. Oxidative Stress and Potential Interventions to Reduce Oxidative Stress in Overweight and Obesity. Diabetes Obes. Metab. 2007, 9, 813–839. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Chen, Z.; Li, C.; Han, T.; Liu, H.; Wang, X. Sestrin2 as a Gatekeeper of Cellular Homeostasis: Physiological Effects for the Regulation of Hypoxia-Related Diseases. J. Cell. Mol. Med. 2021, 25, 5341–5350. [Google Scholar] [CrossRef]
- Chatterjee, S. Chapter Two—Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. ISBN 978-0-12-803269-5. [Google Scholar]
- Verdile, G.; Keane, K.N.; Cruzat, V.F.; Medic, S.; Sabale, M.; Rowles, J.; Wijesekara, N.; Martins, R.N.; Fraser, P.E.; Newsholme, P. Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer’s Disease. Mediat. Inflamm. 2015, 2015, e105828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The Metabolic Syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Wang(b), J.; Wang, S.; Xiao, M.; Zhang, J.; Wang(a), J.; Guo, Y.; Tang, Y.; Gu, J. Regulatory Mechanisms of Sesn2 and Its Role in Multi-Organ Diseases. Pharmacol. Res. 2021, 164, 105331. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; Zheng, Y.; Quan, N. The Emerging Role of Sestrin2 in Cell Metabolism, and Cardiovascular and Age-Related Diseases. Aging Dis. 2020, 11, 154–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Xing, Y.; Xiong, L.; Wang, J. Sestrin2 Overexpression Alleviates Hydrogen Peroxide-Induced Apoptosis and Oxidative Stress in Retinal Ganglion Cells by Enhancing Nrf2 Activation via Keap1 Downregulation. Chem. Biol. Interact. 2020, 324, 109086. [Google Scholar] [CrossRef]
- Park, H.-J.; Yang, S.-G.; Koo, D.-B. SESN2/NRF2 Signaling Activates as a Direct Downstream Regulator of the PERK Pathway against Endoplasmic Reticulum Stress to Improve the in Vitro Maturation of Porcine Oocytes. Free. Radic. Biol. Med. 2022, 178, 413–427. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Du, X.; Huang, Z.; Quan, N. Sestrin 2, a Potential Star of Antioxidant Stress in Cardiovascular Diseases. Free. Radic. Biol. Med. 2021, 163, 56–68. [Google Scholar] [CrossRef]
- Wang, L.; Quan, N.; Sun, W.; Chen, X.; Cates, C.; Rousselle, T.; Zhou, X.; Zhao, X.; Li, J. Cardiomyocyte-Specific Deletion of Sirt1 Gene Sensitizes Myocardium to Ischaemia and Reperfusion Injury. Cardiovasc. Res. 2018, 114, 805–821. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.; Le, T.N.V.; Fedorova, J.; Yang, Y.; Krause-Hauch, M.; Davitt, K.; Zoungrana, L.I.; Fatmi, M.K.; Lesnefsky, E.J.; Li, J.; et al. The Cardiac Dysfunction Caused by Metabolic Alterations in Alzheimer’s Disease. Front. Cardiovasc. Med. 2022, 9, 850538. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.; Li, Y.; Wu, J.; Yu, S.; Zhu, J.; Li, L.; Zhao, Y. Sestrin2 Overexpression Attenuates Focal Cerebral Ischemic Injury in Rat by Increasing Nrf2/HO-1 Pathway-Mediated Angiogenesis. Neuroscience 2019, 410, 140–149. [Google Scholar] [CrossRef]
- Zhang, N.; Liao, H.-H.; Feng, H.; Mou, S.-Q.; Li, W.-J.; Aiyasiding, X.; Lin, Z.; Ding, W.; Zhou, Z.-Y.; Yan, H.; et al. Knockout of AMPKα2 Blocked the Protection of Sestrin2 Overexpression Against Cardiac Hypertrophy Induced by Pressure Overload. Front. Pharmacol. 2021, 12, 716884. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.; Unni, S.; Krishnappa, G.; Padmanabhan, B. The Keap1–Nrf2 Pathway: Promising Therapeutic Target to Counteract ROS-Mediated Damage in Cancers and Neurodegenerative Diseases. Biophys. Rev. 2017, 9, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Zoungrana, L.I.; Krause-Hauch, M.; Wang, H.; Fatmi, M.K.; Bates, L.; Li, Z.; Kulkarni, P.; Ren, D.; Li, J. The Interaction of MTOR and Nrf2 in Neurogenesis and Its Implication in Neurodegenerative Diseases. Cells 2022, 11, 2048. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.Y.; Jin, S.H.; Cho, I.J.; Ki, S.H. Nrf2-ARE Pathway Regulates Induction of Sestrin-2 Expression. Free. Radic. Biol. Med. 2012, 53, 834–841. [Google Scholar] [CrossRef]
- Bae, S.H.; Sung, S.H.; Oh, S.Y.; Lim, J.M.; Lee, S.K.; Park, Y.N.; Lee, H.E.; Kang, D.; Rhee, S.G. Sestrins Activate Nrf2 by Promoting P62-Dependent Autophagic Degradation of Keap1 and Prevent Oxidative Liver Damage. Cell Metab. 2013, 17, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Frangogiannis, N.G. Cardiac Fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Wynn, T. Cellular and Molecular Mechanisms of Fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Dobaczewski, M.; Frangogiannis, N.G. Chemokines and Cardiac Fibrosis. Front. Biosci. 2009, 1, 391–405. [Google Scholar] [CrossRef]
- Cavalera, M.; Wang, J.; Frangogiannis, N.G. Obesity, Metabolic Dysfunction, and Cardiac Fibrosis: Pathophysiological Pathways, Molecular Mechanisms, and Therapeutic Opportunities. Transl. Res. 2014, 164, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Felisbino, M.B.; McKinsey, T.A. Epigenetics in Cardiac Fibrosis. JACC Basic Transl. Sci. 2018, 3, 704–715. [Google Scholar] [CrossRef]
- Ren, D.; Fedorova, J.; Davitt, K.; Le, T.N.V.; Griffen, J.H.; Liaw, P.C.; Esmon, C.T.; Rezaie, A.R.; Li, J. Activated Protein C Strengthens Cardiac Tolerance to Ischemic Insults in Aging | Circulation Research. Circ. Res. 2022, 130, 252–272. [Google Scholar] [CrossRef] [PubMed]
- Aurigemma, G.P.; de Simone, G.; Fitzgibbons, T.P. Cardiac Remodeling in Obesity. Circ. Cardiovasc. Imaging 2013, 6, 142–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Pan, H.; Tan, H.; Yu, Y. High Free Fatty Acids Level Related with Cardiac Dysfunction in Obese Rats. Diabetes Res. Clin. Pract. 2012, 95, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, M.; Sun, M.; Zhang, Y.; Li, X.; Sun, W.; Quan, N. Sestrin2 Is an Endogenous Antioxidant That Improves Contractile Function in the Heart during Exposure to Ischemia and Reperfusion Stress. Free. Radic. Biol. Med. 2021, 165, 385–394. [Google Scholar] [CrossRef]
- Kim, K.M.; Yang, J.H.; Shin, S.M.; Cho, I.J.; Ki, S.H. Sestrin2: A Promising Therapeutic Target for Liver Diseases. Biol. Pharm. Bull. 2015, 38, 966–970. [Google Scholar] [CrossRef] [Green Version]
- De Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial Dysfunction in Obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Zorzano, A.; Liesa, M.; Palacín, M. Role of Mitochondrial Dynamics Proteins in the Pathophysiology of Obesity and Type 2 Diabetes. Int. J. Biochem. Cell Biol. 2009, 41, 1846–1854. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krause-Hauch, M.; Fedorova, J.; Zoungrana, L.I.; Wang, H.; Fatmi, M.K.; Li, Z.; Iglesias, M.; Slotabec, L.; Li, J. Targeting on Nrf2/Sesn2 Signaling to Rescue Cardiac Dysfunction during High-Fat Diet-Induced Obesity. Cells 2022, 11, 2614. https://doi.org/10.3390/cells11162614
Krause-Hauch M, Fedorova J, Zoungrana LI, Wang H, Fatmi MK, Li Z, Iglesias M, Slotabec L, Li J. Targeting on Nrf2/Sesn2 Signaling to Rescue Cardiac Dysfunction during High-Fat Diet-Induced Obesity. Cells. 2022; 11(16):2614. https://doi.org/10.3390/cells11162614
Chicago/Turabian StyleKrause-Hauch, Meredith, Julia Fedorova, Linda Ines Zoungrana, Hao Wang, Mohammad Kasim Fatmi, Zehui Li, Migdalia Iglesias, Lily Slotabec, and Ji Li. 2022. "Targeting on Nrf2/Sesn2 Signaling to Rescue Cardiac Dysfunction during High-Fat Diet-Induced Obesity" Cells 11, no. 16: 2614. https://doi.org/10.3390/cells11162614