Short-Chain Fatty Acids Impair Neutrophil Antiviral Function in an Age-Dependent Manner
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Neutrophil Isolation from Human Peripheral Blood and Treatment with SCFAs
2.3. Determination of Neutrophil Phenotype by Flow Cytometry
2.4. Migration Assay
2.5. Generation of GFP-Labeled VLPs
2.6. Time-Lapse Microscopy of NETs
2.7. HIV Stimulation
2.8. Quantification of Cytokines and Chemokines by Luminex
2.9. ELISA
2.10. Quantification of GPR43 by Western Blot
2.11. Statistical Analysis
3. Results
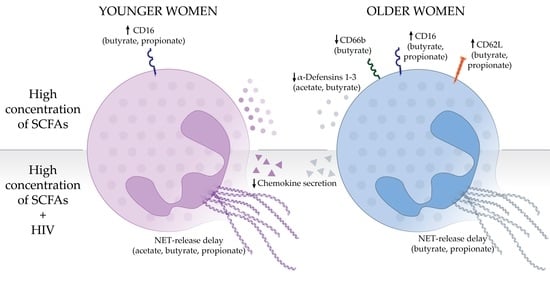
3.1. Pathological Concentrations of SCFAs Induce Phenotypical Changes in Human Blood Neutrophils
3.2. Effects of SCFA Treatment Are Enhanced in Neutrophils from Older Women
3.3. Pathological Concentrations of SCFAs Inhibit Neutrophil Migration In Vitro
3.4. High Concentrations of SCFAs Modify Innate Secretion Profile by Neutrophils from Older Women
3.5. High Concentrations of SCFAs Delay NET Release and Chemokine Secretion in Response to HIV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. HIV/AIDS. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 1 January 2022).
- UNAIDS. Women and Girls and HIV; UNAIDS: Geneva, Switzerland, 2018. [Google Scholar]
- Govender, K.; Beckett, S.; Reddy, T.; Cowden, R.G.; Cawood, C.; Khanyile, D.; Kharsany, A.B.M.; George, G.; Puren, A. Association of HIV Intervention Uptake with HIV Prevalence in Adolescent Girls and Young Women in South Africa. JAMA Netw. Open 2022, 5, e228640. [Google Scholar] [CrossRef]
- CDC. Diagnoses of HIV infection among adults aged 50 years and older in the United States and dependent areas, 2010–2014. In Surveillance Supplemental Report; CDC: Atlanta, GA, USA, 2016; Volume 21. [Google Scholar]
- Tavoschi, L.; Gomes Dias, J.; Pharris, A. New HIV diagnoses among adults aged 50 years or older in 31 European countries, 2004–2015: An analysis of surveillance data. Lancet HIV 2017, 4, e514–e521. [Google Scholar] [CrossRef]
- He, W.; Goodkind, D.; Kowal, P. An Aging World: 2015; US Census Bureau: Suitland, MD, USA, 2016. [Google Scholar]
- Rodriguez-Garcia, M.; Patel, M.V.; Shen, Z.; Wira, C.R. The impact of aging on innate and adaptive immunity in the human female genital tract. Aging Cell 2021, 20, e13361. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Diaz, D.J.; Tyssen, D.; Hayward, J.A.; Gugasyan, R.; Hearps, A.C.; Tachedjian, G. Distinct Immune Responses Elicited From Cervicovaginal Epithelial Cells by Lactic Acid and Short Chain Fatty Acids Associated With Optimal and Non-optimal Vaginal Microbiota. Front. Cell. Infect. Microbiol. 2019, 9, 446. [Google Scholar] [CrossRef]
- Gustin, A.; Cromarty, R.; Schifanella, L.; Klatt, N.R. Microbial mismanagement: How inadequate treatments for vaginal dysbiosis drive the HIV epidemic in women. Semin. Immunol. 2021, 51, 101482. [Google Scholar] [CrossRef]
- Al-Mushrif, S.; Eley, A.; Jones, B.M. Inhibition of chemotaxis by organic acids from anaerobes may prevent a purulent response in bacterial vaginosis. J. Med. Microbiol. 2000, 49, 1023–1030. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Gosmann, C.; Anahtar, M.N.; Handley, S.A.; Farcasanu, M.; Abu-Ali, G.; Bowman, B.A.; Padavattan, N.; Desai, C.; Droit, L.; Moodley, A.; et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity 2017, 46, 29–37. [Google Scholar] [CrossRef]
- Tyssen, D.; Wang, Y.Y.; Hayward, J.A.; Agius, P.A.; DeLong, K.; Aldunate, M.; Ravel, J.; Moench, T.R.; Cone, R.A.; Tachedjian, G. Anti-HIV-1 Activity of Lactic Acid in Human Cervicovaginal Fluid. mSphere 2018, 3, e00055-18. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Brown, C.J.; Abdo, Z.; Davis, C.C.; Hansmann, M.A.; Joyce, P.; Foster, J.A.; Forney, L.J. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007, 1, 121–133. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, L.R.; Achilles, S.L.; Bradshaw, C.S.; Burgener, A.; Crucitti, T.; Fredricks, D.N.; Jaspan, H.B.; Kaul, R.; Kaushic, C.; Klatt, N.; et al. The Evolving Facets of Bacterial Vaginosis: Implications for HIV Transmission. AIDS Res. Hum. Retrovir. 2019, 35, 219–228. [Google Scholar] [CrossRef]
- Eastment, M.C.; McClelland, R.S. Vaginal microbiota and susceptibility to HIV. AIDS 2018, 32, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Sewankambo, N.; Gray, R.H.; Wawer, M.J.; Paxton, L.; McNaim, D.; Wabwire-Mangen, F.; Serwadda, D.; Li, C.; Kiwanuka, N.; Hillier, S.L.; et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 1997, 350, 546–550. [Google Scholar] [CrossRef]
- Salazar, A.S.; Nogueira, N.F.; Rodriguez, V.J.; Mantero, A.; Cherenack, E.M.; Raccamarich, P.; Maddalon, M.; Brophy, T.; Montgomerie, E.; Klatt, N.R.; et al. A Syndemic Approach to Explore Factors Associated with Bacterial Vaginosis. AIDS Behav. 2022, 26, 3110–3118. [Google Scholar] [CrossRef]
- Low, N.; Chersich, M.F.; Schmidlin, K.; Egger, M.; Francis, S.C.; van de Wijgert, J.H.; Hayes, R.J.; Baeten, J.M.; Brown, J.; Delany-Moretlwe, S.; et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: Individual participant data meta-analysis. PLoS Med. 2011, 8, e1000416. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, N.; Singhal, N.; Kaur, R.; Manektala, U. Vaginal microflora in postmenopausal women on hormone replacement therapy. Indian J. Pathol. Microbiol. 2006, 49, 457–461. [Google Scholar]
- Thurman, A.R.; Yousefieh, N.; Chandra, N.; Kimble, T.; Asin, S.; Rollenhagen, C.; Anderson, S.M.; Herold, B.C.; Freiermuth, J.L.; Starkman, B.S.; et al. Comparison of Mucosal Markers of Human Immunodeficiency Virus Susceptibility in Healthy Premenopausal versus Postmenopausal Women. AIDS Res. Hum. Retrovir. 2017, 33, 807–819. [Google Scholar] [CrossRef]
- Hudson, P.L.; Ling, W.; Wu, M.C.; Hayward, M.R.; Mitchell, A.J.; Larson, J.; Guthrie, K.A.; Reed, S.D.; Kwon, D.S.; Mitchell, C.M. Comparison of the Vaginal Microbiota in Postmenopausal Black and White Women. J. Infect. Dis. 2021, 224, 1945–1949. [Google Scholar] [CrossRef]
- Jais, M.; Younes, N.; Chapman, S.; Cu-Uvin, S.; Ghosh, M. Reduced levels of genital tract immune biomarkers in postmenopausal women: Implications for HIV acquisition. Am. J. Obs. Gynecol. 2016, 215, 324.e1–324.e10. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, M.; Connors, K.; Ghosh, M. HIV Pathogenesis in the Human Female Reproductive Tract. Curr. HIV/AIDS Rep. 2021, 18, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, M.; Barr, F.D.; Crist, S.G.; Fahey, J.V.; Wira, C.R. Phenotype and susceptibility to HIV infection of CD4+ Th17 cells in the human female reproductive tract. Mucosal Immunol. 2014, 7, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- De Lara, L.M.; Parthasarathy, R.S.; Rodriguez-Garcia, M. Mucosal Immunity and HIV Acquisition in Women. Curr. Opin. Physiol. 2021, 19, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Chaudry, A.N.; Travers, P.J.; Yuenger, J.; Colletta, L.; Evans, P.; Zenilman, J.M.; Tummon, A. Analysis of vaginal acetic acid in patients undergoing treatment for bacterial vaginosis. J. Clin. Microbiol. 2004, 42, 5170–5175. [Google Scholar] [CrossRef]
- Mirmonsef, P.; Gilbert, D.; Zariffard, M.R.; Hamaker, B.R.; Kaur, A.; Landay, A.L.; Spear, G.T. The effects of commensal bacteria on innate immune responses in the female genital tract. Am. J. Reprod. Immunol. 2011, 65, 190–195. [Google Scholar] [CrossRef]
- Mirmonsef, P.; Zariffard, M.R.; Gilbert, D.; Makinde, H.; Landay, A.L.; Spear, G.T. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. Am. J. Reprod. Immunol. 2012, 67, 391–400. [Google Scholar] [CrossRef]
- Baldewijns, S.; Sillen, M.; Palmans, I.; Vandecruys, P.; Van Dijck, P.; Demuyser, L. The Role of Fatty Acid Metabolites in Vaginal Health and Disease: Application to Candidiasis. Front. Microbiol. 2021, 12, 705779. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Ahmed, K.; Tunaru, S.; Offermanns, S. GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol. Sci. 2009, 30, 557–562. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front. Immunol. 2020, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Dabee, S.; Passmore, J.S.; Heffron, R.; Jaspan, H.B. The Complex Link between the Female Genital Microbiota, Genital Infections, and Inflammation. Infect. Immun. 2021, 89, e00487-20. [Google Scholar] [CrossRef]
- Selhorst, P.; Masson, L.; Ismail, S.D.; Samsunder, N.; Garrett, N.; Mansoor, L.E.; Abdool Karim, Q.; Abdool Karim, S.S.; Passmore, J.S.; Williamson, C. Cervicovaginal Inflammation Facilitates Acquisition of Less Infectious HIV Variants. Clin. Infect. Dis. 2017, 64, 79–82. [Google Scholar] [CrossRef]
- Barr, F.D.; Ochsenbauer, C.; Wira, C.R.; Rodriguez-Garcia, M. Neutrophil extracellular traps prevent HIV infection in the female genital tract. Mucosal Immunol. 2018, 11, 1420–1428. [Google Scholar] [CrossRef]
- Saitoh, T.; Komano, J.; Saitoh, Y.; Misawa, T.; Takahama, M.; Kozaki, T.; Uehata, T.; Iwasaki, H.; Omori, H.; Yamaoka, S.; et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 2012, 12, 109–116. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Arnold, K.B.; Burgener, A.; Birse, K.; Romas, L.; Dunphy, L.J.; Shahabi, K.; Abou, M.; Westmacott, G.R.; McCorrister, S.; Kwatampora, J.; et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol. 2016, 9, 194–205. [Google Scholar] [CrossRef]
- Fan, S.R.; Liu, X.P.; Liao, Q.P. Human defensins and cytokines in vaginal lavage fluid of women with bacterial vaginosis. Int. J. Gynaecol. Obs. 2008, 103, 50–54. [Google Scholar] [CrossRef]
- Levinson, P.; Kaul, R.; Kimani, J.; Ngugi, E.; Moses, S.; MacDonald, K.S.; Broliden, K.; Hirbod, T.; Kibera, H.I.V.S.G. Levels of innate immune factors in genital fluids: Association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS 2009, 23, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Forthal, D.N.; Landucci, G.; Ding, H.; Kappes, J.C.; Wang, A.; Thung, I.; Phan, T. IgG2 inhibits HIV-1 internalization by monocytes, and IgG subclass binding is affected by gp120 glycosylation. AIDS 2011, 25, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Svehla, K.; Mathy, N.L.; Voss, G.; Mascola, J.R.; Wyatt, R. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J. Virol. 2006, 80, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Gartner, S.; Markovits, P.; Markovitz, D.M.; Kaplan, M.H.; Gallo, R.C.; Popovic, M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 1986, 233, 215–219. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.; Biswas, N.; Patel, M.V.; Barr, F.D.; Crist, S.G.; Ochsenbauer, C.; Fahey, J.V.; Wira, C.R. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV-infection. PLoS ONE 2013, 8, e62069. [Google Scholar] [CrossRef]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on Neutrophil Function in Severe Inflammation. Front. Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef]
- Uhl, B.; Vadlau, Y.; Zuchtriegel, G.; Nekolla, K.; Sharaf, K.; Gaertner, F.; Massberg, S.; Krombach, F.; Reichel, C.A. Aged neutrophils contribute to the first line of defense in the acute inflammatory response. Blood 2016, 128, 2327–2337. [Google Scholar] [CrossRef]
- Schnoor, M.; Alcaide, P.; Voisin, M.B.; van Buul, J.D. Crossing the Vascular Wall: Common and Unique Mechanisms Exploited by Different Leukocyte Subsets during Extravasation. Mediat. Inflamm. 2015, 2015, 946509. [Google Scholar] [CrossRef]
- Lee, D.; Schultz, J.B.; Knauf, P.A.; King, M.R. Mechanical shedding of L-selectin from the neutrophil surface during rolling on sialyl Lewis x under flow. J. Biol. Chem. 2007, 282, 4812–4820. [Google Scholar] [CrossRef]
- Chang, T.L.; Vargas, J., Jr.; DelPortillo, A.; Klotman, M.E. Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. J. Clin. Investig. 2005, 115, 765–773. [Google Scholar] [CrossRef]
- Yeoman, C.J.; Thomas, S.M.; Miller, M.E.; Ulanov, A.V.; Torralba, M.; Lucas, S.; Gillis, M.; Cregger, M.; Gomez, A.; Ho, M.; et al. A multi-omic systems-based approach reveals metabolic markers of bacterial vaginosis and insight into the disease. PLoS ONE 2013, 8, e56111. [Google Scholar] [CrossRef] [PubMed]
- Vitali, B.; Cruciani, F.; Picone, G.; Parolin, C.; Donders, G.; Laghi, L. Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Masson, L.; Mlisana, K.; Little, F.; Werner, L.; Mkhize, N.N.; Ronacher, K.; Gamieldien, H.; Williamson, C.; McKinnon, L.R.; Walzl, G.; et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: A cross-sectional study. Sex. Transm. Infect. 2014, 90, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Morgan, M.T.; Fiedler, T.L.; Djukovic, D.; Hoffman, N.G.; Raftery, D.; Marrazzo, J.M.; Fredricks, D.N. Metabolic signatures of bacterial vaginosis. MBio 2015, 6, e00204-15. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Terada, A.; Kita, H. CD66b regulates adhesion and activation of human eosinophils. J. Immunol. 2007, 179, 8454–8462. [Google Scholar] [CrossRef]
- Dransfield, I.; Buckle, A.M.; Savill, J.S.; McDowall, A.; Haslett, C.; Hogg, N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. J. Immunol. 1994, 153, 1254–1263. [Google Scholar]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Chevre, R.; A-González, N.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, G.; Manwani, D.; Mortha, A.; Xu, C.; Faith, J.J.; Burk, R.D.; Kunisaki, Y.; Jang, J.E.; Scheiermann, C.; et al. Neutrophil ageing is regulated by the microbiome. Nature 2015, 525, 528–532. [Google Scholar] [CrossRef]
- Beghini, J.; Giraldo, P.C.; Riboldi, R.; Amaral, R.L.; Eleuterio, J., Jr.; Witkin, S.S.; Guimaraes, F. Altered CD16 expression on vaginal neutrophils from women with vaginitis. Eur. J. Obs. Gynecol. Reprod. Biol. 2013, 167, 96–99. [Google Scholar] [CrossRef]
- Moulding, D.; Quayle, J.A.; Stringer, R.E.; Hart, C.A.; Edwards, S.W. Regulation of neutrophil apoptosis by sodium butyrate. Biologicals 1996, 24, 301–306. [Google Scholar] [CrossRef]
- Aoyama, M.; Kotani, J.; Usami, M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 2010, 26, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Hidalgo, A.; Soehnlein, O. Neutrophil heterogeneity: Implications for homeostasis and pathogenesis. Blood 2016, 127, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Jenne, C.N.; Surewaard, B.G.; Thanabalasuriar, A.; Lee, W.Y.; Sanz, M.J.; Mowen, K.; Opdenakker, G.; Kubes, P. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat. Commun. 2015, 6, 6673. [Google Scholar] [CrossRef] [PubMed]
- Farr, C.; Noel-Romas, L.; Birse, K.; De Leon, M.; Kratzer, K.; Perner, M.; Ayele, H.; Wiwchar, C.; Pymar, H.; Berard, A.R.; et al. Increased cervical neutrophil survival during bacterial vaginosis in Canadian women from the THRIVE study. J. Immunol. 2020, 204, 157.13. [Google Scholar]
- Kahn, J.; Ingraham, R.H.; Shirley, F.; Migaki, G.I.; Kishimoto, T.K. Membrane proximal cleavage of L-selectin: Identification of the cleavage site and a 6-kD transmembrane peptide fragment of L-selectin. J. Cell. Biol. 1994, 125, 461–470. [Google Scholar] [CrossRef]
- Ivetic, A. A head-to-tail view of L-selectin and its impact on neutrophil behaviour. Cell. Tissue Res. 2018, 371, 437–453. [Google Scholar] [CrossRef]
- Schleiffenbaum, B.; Spertini, O.; Tedder, T.F. Soluble L-selectin is present in human plasma at high levels and retains functional activity. J. Cell. Biol. 1992, 119, 229–238. [Google Scholar] [CrossRef]
- Ivetic, A.; Hoskins Green, H.L.; Hart, S.J. L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front. Immunol. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Correa, R.O.; Vieira, A.; Sernaglia, E.M.; Lancellotti, M.; Vieira, A.T.; Avila-Campos, M.J.; Rodrigues, H.G.; Vinolo, M.A.R. Bacterial short-chain fatty acid metabolites modulate the inflammatory response against infectious bacteria. Cell. Microbiol. 2017, 19, e12720. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Hebeda, C.B.; Farsky, S.H.; Curi, R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. 2009, 117, 331–338. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Ferguson, G.J.; Kulkarni, S.; Damoulakis, G.; Anderson, K.; Bohlooly, Y.M.; Stephens, L.; Hawkins, P.T.; Curi, R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS ONE 2011, 6, e21205. [Google Scholar] [CrossRef] [PubMed]
- Sina, C.; Gavrilova, O.; Forster, M.; Till, A.; Derer, S.; Hildebrand, F.; Raabe, B.; Chalaris, A.; Scheller, J.; Rehmann, A.; et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 2009, 183, 7514–7522. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. Mechanistic Insights into Immune Suppression and Evasion in Bacterial Vaginosis. Curr. Microbiol. 2022, 79, 84. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Hatanaka, E.; Lambertucci, R.H.; Newsholme, P.; Curi, R. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell. Biochem. Funct. 2009, 27, 48–55. [Google Scholar] [CrossRef]
- Klotman, M.E.; Chang, T.L. Defensins in innate antiviral immunity. Nat. Rev. Immunol. 2006, 6, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, M.; Climent, N.; Oliva, H.; Casanova, V.; Franco, R.; Leon, A.; Gatell, J.M.; Garcia, F.; Gallart, T. Increased alpha-defensins 1-3 production by dendritic cells in HIV-infected individuals is associated with slower disease progression. PLoS ONE 2010, 5, e9436. [Google Scholar] [CrossRef] [PubMed]
- Iniguez-Gutierrez, L.; Godinez-Mendez, L.A.; Fafutis-Morris, M.; Padilla-Arellano, J.R.; Corona-Rivera, A.; Bueno-Topete, M.R.; Rojas-Rejon, O.A.; Delgado-Rizo, V. Physiological concentrations of short-chain fatty acids induce the formation of neutrophil extracellular traps in vitro. Int. J. Immunopathol. Pharm. 2020, 34, 2058738420958949. [Google Scholar] [CrossRef]
- Andany, N.; Kennedy, V.L.; Aden, M.; Loutfy, M. Perspectives on menopause and women with HIV. Int. J. Womens Health 2016, 8, 1–22. [Google Scholar] [CrossRef]
- Iqbal, A.J.; Krautter, F.; Blacksell, I.A.; Wright, R.D.; Austin-Williams, S.N.; Voisin, M.B.; Hussain, M.T.; Law, H.L.; Niki, T.; Hirashima, M.; et al. Galectin-9 mediates neutrophil capture and adhesion in a CD44 and beta2 integrin-dependent manner. FASEB J. 2022, 36, e22065. [Google Scholar] [CrossRef] [PubMed]
- Fichorova, R.N.; DeLong, A.K.; Cu-Uvin, S.; King, C.C.; Jamieson, D.J.; Klein, R.S.; Sobel, J.D.; Vlahov, D.; Yamamoto, H.S.; Mayer, K.H. Protozoan-Viral-Bacterial Co-Infections Alter Galectin Levels and Associated Immunity Mediators in the Female Genital Tract. Front. Cell. Infect. Microbiol. 2021, 11, 649940. [Google Scholar] [CrossRef]
- Dunsmore, G.; Rosero, E.P.; Shahbaz, S.; Santer, D.M.; Jovel, J.; Lacy, P.; Houston, S.; Elahi, S. Neutrophils promote T-cell activation through the regulated release of CD44-bound Galectin-9 from the cell surface during HIV infection. PLoS Biol. 2021, 19, e3001387. [Google Scholar] [CrossRef] [PubMed]
- Bozorgmehr, N.; Mashhouri, S.; Perez Rosero, E.; Xu, L.; Shahbaz, S.; Sligl, W.; Osman, M.; Kutsogiannis, D.J.; MacIntyre, E.; O’Neil, C.R.; et al. Galectin-9, a Player in Cytokine Release Syndrome and a Surrogate Diagnostic Biomarker in SARS-CoV-2 Infection. MBio 2021, 12, e00384-21. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Salinas, F.J.; Parthasarathy, S.; Moreno de Lara, L.; Borchers, A.; Ochsenbauer, C.; Panda, A.; Rodriguez-Garcia, M. Short-Chain Fatty Acids Impair Neutrophil Antiviral Function in an Age-Dependent Manner. Cells 2022, 11, 2515. https://doi.org/10.3390/cells11162515
Carrillo-Salinas FJ, Parthasarathy S, Moreno de Lara L, Borchers A, Ochsenbauer C, Panda A, Rodriguez-Garcia M. Short-Chain Fatty Acids Impair Neutrophil Antiviral Function in an Age-Dependent Manner. Cells. 2022; 11(16):2515. https://doi.org/10.3390/cells11162515
Chicago/Turabian StyleCarrillo-Salinas, Francisco J., Siddharth Parthasarathy, Laura Moreno de Lara, Anna Borchers, Christina Ochsenbauer, Alexander Panda, and Marta Rodriguez-Garcia. 2022. "Short-Chain Fatty Acids Impair Neutrophil Antiviral Function in an Age-Dependent Manner" Cells 11, no. 16: 2515. https://doi.org/10.3390/cells11162515