Inhibition of the Combinatorial Signaling of Transforming Growth Factor-Beta and NOTCH Promotes Myotube Formation of Human Pluripotent Stem Cell-Derived Skeletal Muscle Progenitor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture of hiPSCs
2.2. In Vitro Skeletal Muscle Differentiation
2.3. Isolation of NCAM+/HNK- Myoblasts from hiPSCs Derived Differentiated Cells
2.4. Cell Culture of Myoblasts and Myotube Formation
2.5. Small Molecule Screening for Enhancing Myotube Formation
2.6. Growth Curve Assay
2.7. Cell Proliferation and Apoptosis Assay
2.8. Immunofluorescence Analysis
2.9. Fusion Index and Ratio of Area Analysis
2.10. Quantitative Real-Time PCR Analysis
2.11. Statistical Analysis
3. Results
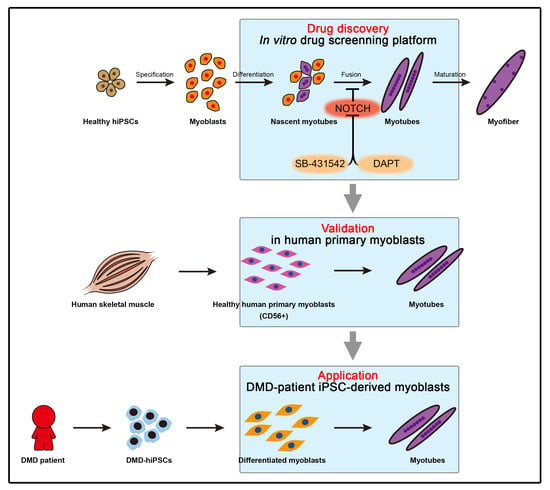
3.1. Establishment of the Screening Platform Based on hPSC-Derived Skeletal Muscle Differentiation
3.2. Small-Scale Screening for Small Molecules Enhancing In Vitro Myotube Formation
3.3. Improved Efficiency of Myotube Formation by Combinational Treatment
3.4. Physiological Role of TGFβ and NOTCH during Myogenesis
3.5. Application of Small Molecules Enhances Myotube Formation of Primary Myoblasts and Patient’s Myoblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charge, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Pannerec, A.; Cadot, B.; Parlakian, A.; Besson, V.; Gomes, E.R.; Marazzi, G.; Sassoon, D.A. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 2010, 12, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff, R. Regeneration of single skeletal muscle fibers in vitro. Anat. Rec. 1975, 182, 215–235. [Google Scholar] [CrossRef]
- Konigsberg, I.R. Cellular differentiation in colonies derived from single cells platings of freshly isolated chick embryo muscle cells. Proc. Natl. Acad. Sci. USA 1961, 47, 1868–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, J.W.; Capel, A.J.; Rimington, R.P.; Wheeler, P.; Leonard, A.N.; Bishop, N.C.; Davies, O.G.; Lewis, M.P. Bioengineered human skeletal muscle capable of functional regeneration. BMC Biol. 2020, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Wilschut, K.J.; Ling, V.B.; Bernstein, H.S. Concise review: Stem cell therapy for muscular dystrophies. Stem. Cells Transl. Med. 2012, 1, 833–842. [Google Scholar] [CrossRef]
- Bajek, A.; Porowinska, D.; Kloskowski, T.; Brzoska, E.; Ciemerych, M.A.; Drewa, T. Cell therapy in Duchenne muscular dystrophy treatment: Clinical trials overview. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 1–11. [Google Scholar] [CrossRef]
- Ryder, S.; Leadley, R.M.; Armstrong, N.; Westwood, M.; de Kock, S.; Butt, T.; Jain, M.; Kleijnen, J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: An evidence review. Orphanet J. Rare Dis. 2017, 12, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, I.Y.; Lim, H.; Estrellas, K.; Mula, J.; Cohen, T.V.; Zhang, Y.; Donnelly, C.J.; Richard, J.P.; Kim, Y.J.; Kim, H.; et al. Concordant but Varied Phenotypes among Duchenne Muscular Dystrophy Patient-Specific Myoblasts Derived using a Human iPSC-Based Model. Cell Rep. 2016, 15, 2301–2312. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.Y.; Lim, H.; Lee, G. Efficient generation human induced pluripotent stem cells from human somatic cells with Sendai-virus. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.Y.; Lim, H.; Cho, H.J.; Oh, Y.; Chou, B.K.; Bai, H.; Cheng, L.; Kim, Y.J.; Hyun, S.; Kim, H.; et al. Transcriptional landscape of myogenesis from human pluripotent stem cells reveals a key role of TWIST1 in maintenance of skeletal muscle progenitors. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.I.; Chen, J.C.; Rahimov, F.; Homma, S.; Arashiro, P.; Beermann, M.L.; King, O.D.; Miller, J.B.; Kunkel, L.M.; Emerson, C.P., Jr.; et al. Facioscapulohumeral muscular dystrophy family studies of DUX4 expression: Evidence for disease modifiers and a quantitative model of pathogenesis. Hum. Mol. Genet. 2012, 21, 4419–4430. [Google Scholar] [CrossRef] [Green Version]
- Stadler, G.; Chen, J.C.; Wagner, K.; Robin, J.D.; Shay, J.W.; Emerson, C.P., Jr.; Wright, W.E. Establishment of clonal myogenic cell lines from severely affected dystrophic muscles—CDK4 maintains the myogenic population. Skelet. Muscle 2011, 1, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Lim, H.; Li, Z.; Oh, Y.; Kovlyagina, I.; Choi, I.Y.; Dong, X.; Lee, G. Generation of multipotent induced neural crest by direct reprogramming of human postnatal fibroblasts with a single transcription factor. Cell Stem. Cell 2014, 15, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [Green Version]
- Edom-Vovard, F.; Mouly, V.; Barbet, J.P.; Butler-Browne, G.S. The four populations of myoblasts involved in human limb muscle formation are present from the onset of primary myotube formation. J. Cell Sci. 1999, 112 Pt. 2, 191–199. [Google Scholar] [CrossRef]
- Hicks, M.R.; Hiserodt, J.; Paras, K.; Fujiwara, W.; Eskin, A.; Jan, M.; Xi, H.; Young, C.S.; Evseenko, D.; Nelson, S.F.; et al. ERBB3 and NGFR mark a distinct skeletal muscle progenitor cell in human development and hPSCs. Nat. Cell Biol. 2018, 20, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, S.; Mondragon-Gonzalez, R.; Xu, B.; Magli, A.; Kim, H.; Laine, J.; Kiley, J.; McKee, H.; Rinaldi, F.; Aho, J.; et al. Screening identifies small molecules that enhance the maturation of human pluripotent stem cell-derived myotubes. Elife 2019, 8. [Google Scholar] [CrossRef]
- Sun, C.; Choi, I.Y.; Rovira Gonzalez, Y.I.; Andersen, P.; Talbot, C.C., Jr.; Iyer, S.R.; Lovering, R.M.; Wagner, K.R.; Lee, G. Duchenne muscular dystrophy hiPSC-derived myoblast drug screen identifies compounds that ameliorate disease in mdx mice. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Schachtrup, C.; Ryu, J.K.; Mammadzada, K.; Khan, A.S.; Carlton, P.M.; Perez, A.; Christian, F.; Le Moan, N.; Vagena, E.; Baeza-Raja, B.; et al. Nuclear pore complex remodeling by p75(NTR) cleavage controls TGF-beta signaling and astrocyte functions. Nat. Neurosci. 2015, 18, 1077–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Xue, S.; Zhou, J.; Voorhees, J.J.; Fisher, G.J. Notch and TGF-beta pathways cooperatively regulate receptor protein tyrosine phosphatase-kappa (PTPRK) gene expression in human primary keratinocytes. Mol. Biol. Cell 2015, 26, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Ismaeel, A.; Kim, J.S.; Kirk, J.S.; Smith, R.S.; Bohannon, W.T.; Koutakis, P. Role of Transforming Growth Factor-β in Skeletal Muscle Fibrosis: A Review. Int. J. Mol. Sci. 2019, 20, 2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blokzijl, A.; Dahlqvist, C.; Reissmann, E.; Falk, A.; Moliner, A.; Lendahl, U.; Ibanez, C.F. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J. Cell Biol. 2003, 163, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Garry, D.J. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006, 20, 1692–1708. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Serra, C.; Lee, G.; Wagner, K.R. Stem cell-based therapies for Duchenne muscular dystrophy. Exp. Neurol. 2020, 323, 113086. [Google Scholar] [CrossRef]
- Lagalice, L.; Pichon, J.; Gougeon, E.; Soussi, S.; Deniaud, J.; Ledevin, M.; Maurier, V.; Leroux, I.; Durand, S.; Ciron, C.; et al. Satellite cells fail to contribute to muscle repair but are functional in Pompe disease (glycogenosis type II). Acta Neuropathol. Commun. 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaaf, G.J.; van Gestel, T.J.M.; In’t Groen, S.L.M.; de Jong, B.; Boomaars, B.; Tarallo, A.; Cardone, M.; Parenti, G.; van der Ploeg, A.T.; Pijnappel, W. Satellite cells maintain regenerative capacity but fail to repair disease-associated muscle damage in mice with Pompe disease. Acta Neuropathol. Commun. 2018, 6, 119. [Google Scholar] [CrossRef] [Green Version]
- Kottlors, M.; Kirschner, J. Elevated satellite cell number in Duchenne muscular dystrophy. Cell Tissue Res. 2010, 340, 541–548. [Google Scholar] [CrossRef]
- Liu, D.; Black, B.L.; Derynck, R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001, 15, 2950–2966. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee-Clavin, B.; Mi, R.; Kern, B.; Choi, I.Y.; Lim, H.; Oh, Y.; Lannon, B.; Kim, K.J.; Bell, S.; Hur, J.K.; et al. Comparison of three congruent patient-specific cell types for the modelling of a human genetic Schwann-cell disorder. Nat. Biomed. Eng. 2019, 3, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Mendias, C.L.; Gumucio, J.P.; Davis, M.E.; Bromley, C.W.; Davis, C.S.; Brooks, S.V. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve 2012, 45, 55–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Small Molecule | Full Name | Concentration |
|---|---|---|
| FGFs | FGF2 | 10 ng/mL |
| FGF8 | 100 ng/mL | |
| CHIR | CHIR99021 | 3 uM |
| LY | LY294002 | 1 uM |
| PD | PD173074 | 1 uM |
| LDN | LDN193189 | 50 nM |
| SB | SB431542 | 10 uM |
| PMP | Purmorphamine | 0.5 uM |
| XAV | XAV939 | 2 uM |
| DAPT | DAPT | 10 uM |
| BMP | BMP4 | 2 ng/mL |
| RA | Retinoic Acid | 1 uM |
| TGFs | TGF β1 | 10 ng/mL |
| TGF β2 | 10 ng/mL | |
| TGF β3 | 10 ng/mL | |
| PMA | Phorbol 12-myristate 13-acetate | 10 nM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, I.Y.; Lim, H.T.; Che, Y.H.; Lee, G.; Kim, Y.J. Inhibition of the Combinatorial Signaling of Transforming Growth Factor-Beta and NOTCH Promotes Myotube Formation of Human Pluripotent Stem Cell-Derived Skeletal Muscle Progenitor Cells. Cells 2021, 10, 1649. https://doi.org/10.3390/cells10071649
Choi IY, Lim HT, Che YH, Lee G, Kim YJ. Inhibition of the Combinatorial Signaling of Transforming Growth Factor-Beta and NOTCH Promotes Myotube Formation of Human Pluripotent Stem Cell-Derived Skeletal Muscle Progenitor Cells. Cells. 2021; 10(7):1649. https://doi.org/10.3390/cells10071649
Chicago/Turabian StyleChoi, In Young, Ho Tae Lim, Young Hyun Che, Gabsang Lee, and Yong Jun Kim. 2021. "Inhibition of the Combinatorial Signaling of Transforming Growth Factor-Beta and NOTCH Promotes Myotube Formation of Human Pluripotent Stem Cell-Derived Skeletal Muscle Progenitor Cells" Cells 10, no. 7: 1649. https://doi.org/10.3390/cells10071649