Transcryptomic Analysis of Human Brain-Microvascular Endothelial Response to -Pericytes: Cell Orientation Defines Barrier Function
Abstract
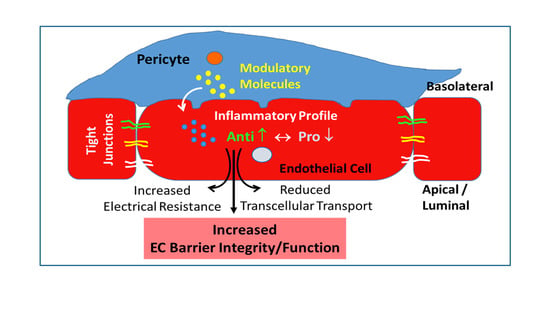
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Barrier Function Studies
2.3. Transendothelial Electric Resistance (TEER)
2.4. Macromolecular Tracer Assay
2.5. Electric Cell Substrate Impedance Sensing (ECIS)
2.6. Conditioned Media (CM)
Co-Culture-Conditioned Media (Co-CM)
2.7. Cell Count
2.8. Crystal Violet Staining (CVS)
2.9. Microarray Analysis
2.10. Cytokine Proteome Array
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
3.1. Preliminary Experiments
3.2. Effect of Different Co-Culture Arrangements on Barrier Function
3.3. Effect of Pericyte-Conditioned Media on Barrier Function
3.4. Gene Expression Array
4. Discussion
4.1. Effect of Co-Culture on Inflammatory Profile of ECs
4.2. Soluble Mediators and Cell Surface Proteins
4.3. Extracellular Matrix Regulation
4.4. Junctional Proteins
4.5. Effects of Pericyte Conditioned Media (PCM) on Endothelial Barrier Function
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armulik, A.; Genove, G.; Mae, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lendahl, U.; Nilsson, P.; Betsholtz, C. Emerging links between cerebrovascular and neurodegenerative diseases—A special role for pericytes. EMBO Rep. 2019, 20, e48070. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Zhou, H.-N.; He, S.-S.; Xue, M.-Y.; Li, T.; Liu, L.-M. Research advances in pericyte function and their roles in diseases. Chin. J. Traumatol. 2020, 23, 89–95. [Google Scholar] [CrossRef]
- Komarova, Y.; Malik, A.B. Regulation of Endothelial Permeability via Paracellular and Transcellular Transport Pathways. Ann. Rev. Physiol. 2010, 72, 463–493. [Google Scholar] [CrossRef]
- Tervonen, A.; Ihalainen, T.O.; Nymark, S.; Hyttinen, J. Structural dynamics of tight junctions modulate the properties of the epithelial barrier. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Hervé, F.; Ghinea, N.; Scherrmann, J.-M. CNS Delivery Via Adsorptive Transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.M.; Goyal, B.R.; Bhadada, S.V.; Bhatt, J.S.; Amin, A.F. Getting into the Brain. CNS Drugs 2009, 23, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Ogunshola, O.O. In Vitro Modeling of the Blood-Brain Barrier: Simplicity Versus Complexity. Curr. Pharm. Des. 2011, 17, 2755–2761. [Google Scholar] [CrossRef] [Green Version]
- Hori, S.; Ohtsuki, S.; Hosoya, K.; Nakashima, E.; Terasaki, T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J. Neurochem. 2004, 89, 503–513. [Google Scholar] [CrossRef]
- Dohgu, S.; Takata, F.; Yamauchi, A.; Nakagawa, S.; Egawa, T.; Naito, M.; Tsuruo, T.; Sawada, Y.; Niwa, M.; Kataoka, Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005, 1038, 208–215. [Google Scholar] [CrossRef]
- McGuire, P.G.; Rangasamy, S.; Maestas, J.; Das, A. Pericyte-Derived Sphinogosine 1-Phosphate Induces the Expression of Adhesion Proteins and Modulates the Retinal Endothelial Cell Barrier. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e107–e115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weksler, B.B.; Subileau, E.A.; Perrière, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005, 19, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.-Y.; Bocci, F.; Jolly, M.K.; Levin, H.; Onuchic, J.N.; Levchenko, A. Pericytes enable effective angiogenesis in the presence of proinflammatory signals. Proc. Natl. Acad. Sci. USA 2019, 116, 23551–23561. [Google Scholar] [CrossRef] [PubMed]
- Hamad, S.; Derichsweiler, D.; Papadopoulos, S.; Nguemo, F.; Saric, T.; Sachinidis, A.; Brockmeier, K.; Hescheler, J.; Boukens, B.J.; Pfannkuche, K. Generation of human induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer and scalable 3D suspension bioreactor cultures with reduced batch-to-batch variations. Theranostics 2019, 9, 7222–7238. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Grishagin, I.; Wang, Y.; Zhao, T.; Greene, J.; Obenauer, J.C.; Ngan, D.; Dac-Trung, N.; Guha, R.; Jadhav, A.; et al. The NCATS BioPlanet—An Integrated Platform for Exploring the Universe of Cellular Signaling Pathways for Toxicology, Systems Biology, and Chemical Genomics. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.L.; Nam, J.-O.; Jean, C.; Lawson, C.; Walsh, C.T.; Goka, E.; Lim, S.-T.; Tomar, A.; Tancioni, I.; Uryu, S.; et al. VEGF-Induced Vascular Permeability Is Mediated by FAK. Dev. Cell 2012, 22, 146–157. [Google Scholar] [CrossRef] [Green Version]
- Schröder-Heurich, B.; Von Hardenberg, S.; Brodowski, L.; Kipke, B.; Meyer, N.; Borns, K.; Von Kaisenberg, C.S.; Brinkmann, H.; Claus, P.; Versen-Höynck, F. Vitamin D improves endothelial barrier integrity and counteracts inflammatory effects on endothelial progenitor cells. FASEB J. 2019, 33, 9142–9153. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, H.; Tai, Z.; Li, T.; Luo, L.; Tong, Z.; Zhu, W. Apigenin and Ethaverine Hydrochloride Enhance Retinal Vascular Barrier In Vitro and In Vivo. Transl. Vis. Sci. Technol. 2020, 9. [Google Scholar] [CrossRef]
- Bednarek, R.; Selmi, A.; Wojkowska, D.; Karolczak, K.; Popielarski, M.; Stasiak, M.; Salifu, M.O.; Babinska, A.; Swiatkowska, M. Functional inhibition of F11 receptor (F11R/junctional adhesion molecule-A/JAM-A) activity by a F11R-derived peptide in breast cancer and its microenvironment. Breast Cancer Res. Treat. 2020, 179, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Deli, M.A.; Kawaguchi, H.; Shimizudani, T.; Shimono, T.; Kittel, A.; Tanaka, K.; Niwa, M. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem. Int. 2009, 54, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, J.J.; Linville, R.M.; Ding, Y.Y.; Gerecht, S.; Searson, P.C. Role of iPSC-derived pericytes on barrier function of iPSC-derived brain microvascular endothelial cells in 2D and 3D. Fluids Barriers CNS 2019, 16, 1–16. [Google Scholar] [CrossRef]
- Kovacs, Z.I.; Kim, S.; Jikaria, N.; Qureshi, F.; Milo, B.; Lewis, B.K.; Bresler, M.; Burks, S.R.; Frank, J.A. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, E75–E84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood–brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Yu, Y.; Qin, J.; Liu, M.Z.; Ruan, Q.Y.; Li, Y.L.; Zhang, Z.H. Role of Rho kinase in lysophosphatidic acid-induced altering of blood-brain barrier permeability. Int. J. Mol. Med. 2014, 33, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Corem, N.; Anzi, S.; Gelb, S.; Ben-Zvi, A. Leptin receptor deficiency induces early, transient and hyperglycaemia-independent blood-brain barrier dysfunction. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Miloudi, K.; Oubaha, M.; Menard, C.; Dejda, A.; Guber, V.; Cagnone, G.; Wilson, A.M.; Tetreault, N.; Mawambo, G.; Binet, F.; et al. NOTCH1 signaling induces pathological vascular permeability in diabetic retinopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 4538–4547. [Google Scholar] [CrossRef] [Green Version]
- Le Roux, G.; Jarray, R.; Guyot, A.-C.; Pavoni, S.; Costa, N.; Theodoro, F.; Nassor, F.; Pruvost, A.; Tournier, N.; Kiyan, Y.; et al. Proof-of-Concept Study of Drug Brain Permeability between In Vivo Human Brain and an In Vitro iPSCs-Human Blood-Brain Barrier Model. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [Green Version]
- Hatherell, K.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Pilkington, G.J. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods 2011, 199, 223–229. [Google Scholar] [CrossRef]
- Klein, O.; Roded, A.; Hirschberg, K.; Fukuda, M.; Galli, S.J.; Sagi-Eisenberg, R. Imaging FITC-dextran as a Reporter for Regulated Exocytosis. J. Vis. Exp. 2018, e57936. [Google Scholar] [CrossRef]
- Lai, C.H.; Kuo, K.H. The critical component to establish in vitro BBB model: Pericyte. Brain Res. Rev. 2005, 50, 258–265. [Google Scholar] [CrossRef]
- Maherally, Z.; Fillmore, H.L.; Tan, S.L.; Tan, S.F.; Jassam, S.A.; Quack, F.I.; Hatherell, K.E.; Pilkington, G.J. Real-time acquisition of transendothelial electrical resistance in an all-human, in vitro, 3-dimensional, blood-brain barrier model exemplifies tight-junction integrity. FASEB J. 2018, 32, 168–182. [Google Scholar] [CrossRef] [Green Version]
- Mobed-Miremadi, M.; Djomehri, S.; Keralapura, M.; McNeil, M. Fickian-Based Empirical Approach for Diffusivity Determination in Hollow Alginate-Based Microfibers Using 2D Fluorescence Microscopy and Comparison with Theoretical Predictions. Materials 2014, 7, 7670–7688. [Google Scholar] [CrossRef] [Green Version]
- Cacopardo, L.; Costa, J.; Giusti, S.; Buoncompagni, L.; Meucci, S.; Corti, A.; Mattei, G.; Ahluwalia, A. Real-time cellular impedance monitoring and imaging of biological barriers in a dual-flow membrane bioreactor. Biosens. Bioelectron. 2019, 140, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, I.; Hornburger, M.; Mayer, B.; Beyerle, A.; Wegener, J.; Furst, R. Pitfalls in assessing microvascular endothelial barrier function: Impedance-based devices versus the classic macromolecular tracer assay. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heymans, M.; Figueiredo, R.; Dehouck, L.; Francisco, D.; Sano, Y.; Shimizu, F.; Kanda, T.; Bruggmann, R.; Engelhardt, B.; Winter, P.; et al. Contribution of brain pericytes in blood–brain barrier formation and maintenance: A transcriptomic study of cocultured human endothelial cells derived from hematopoietic stem cells. Fluids Barriers CNS 2020, 17, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Body-Malapel, M.; Djouina, M.; Waxin, C.; Langlois, A.; Gower-Rousseau, C.; Zerbib, P.; Schmidt, A.M.; Desreumaux, P.; Boulanger, E.; Vignal, C. The RAGE signaling pathway is involved in intestinal inflammation and represents a promising therapeutic target for Inflammatory Bowel Diseases. Mucosal Immunol. 2019, 12, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Birnhuber, A.; Biasin, V.; Schnoegl, D.; Marsh, L.M.; Kwapiszewska, G. Transcription factor Fra-2 and its emerging role in matrix deposition, proliferation and inflammation in chronic lung diseases. Cell. Signal. 2019, 64. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Holman, D.W.; Klein, R.S. Chemokines in the balance: Maintenance of homeostasis and protection at CNS barriers. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Ma, X.; Jiang, D.; Wang, L.; Zhan, Q.; Zhao, J. CXC chemokine ligand 5 (CXCL5) disrupted the permeability of human brain microvascular endothelial cells via regulating p38 signal. Microbiol. Immunol. 2020. [Google Scholar] [CrossRef]
- Haarmann, A.; Schuhmann, M.K.; Silwedel, C.; Monoranu, C.-M.; Stoll, G.; Buttmann, M. Human Brain Endothelial CXCR2 Is Inflammation-Inducible and Mediates CXCL5-and CXCL8-Triggered Paraendothelial Barrier Breakdown. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [Green Version]
- Kovarik, P.; Ebner, F.; Sedlyarov, V. Posttranscriptional regulation of cytokine expression. Cytokine 2017, 89, 21–26. [Google Scholar] [CrossRef]
- Chai, Q.; He, W.Q.; Zhou, M.; Lu, H.; Fu, Z.F. Enhancement of Blood-Brain Barrier Permeability and Reduction of Tight Junction Protein Expression Are Modulated by Chemokines/Cytokines Induced by Rabies Virus Infection. J. Virol. 2014, 88, 4698–4710. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.K.; Ryu, H.J.; Kim, M.-J.; Kim, W.I.; Choi, H.K.; Choi, H.-C.; Song, H.-K.; Jo, S.-M.; Kang, T.-C. Interleukin-18 attenuates disruption of brain-blood barrier induced by status epilepticus within the rat piriform cortex in interferon-gamma independent pathway. Brain Res. 2012, 1447, 126–134. [Google Scholar] [CrossRef]
- Fu, A.K.Y.; Hung, K.-W.; Yuen, M.Y.F.; Zhou, X.; Mak, D.S.Y.; Chan, I.C.W.; Cheung, T.H.; Zhang, B.; Fu, W.-Y.; Liew, F.Y.; et al. IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc. Natl. Acad. Sci. USA 2016, 113, E2705–E2713. [Google Scholar] [CrossRef] [Green Version]
- Reverchon, F.; Mortaud, S.; Sivoyon, M.; Maillet, I.; Laugeray, A.; Palomo, J.; Montécot, C.; Herzine, A.; Meme, S.; Meme, W.; et al. IL-33 receptor ST2 regulates the cognitive impairments associated with experimental cerebral malaria. PLoS Pathog. 2017, 13, e1006322. [Google Scholar] [CrossRef] [Green Version]
- Lotfi, N.; Thome, R.; Rezaei, N.; Zhang, G.X.; Rostami, A.; Esmaeil, N. Roles of GM-CSF in the pathogenesis of autoimmune diseases: An update. Eur. J. Immunol. 2019, 49, 938–939. [Google Scholar] [CrossRef] [PubMed]
- Goebel, K.; Asaridou, C.-M.; Merker, M.; Eichler, S.; Herrmann, A.M.; Geuss, E.; Ruck, T.; Schuengel, L.; Groeneweg, L.; Narayanan, V.; et al. Plasma kallikrein modulates immune cell trafficking during neuroinflammation via PAR2 and bradykinin release. Proc. Natl. Acad. Sci. USA 2019, 116, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Gawlak, G.; Shah, A.S.; Higginbotham, K.; Tian, X.; Kawasaki, Y.; Akiyama, T.; Sacks, D.B.; Birukova, A.A. Hepatocyte Growth Factor-induced Asef-IQGAP1 Complex Controls Cytoskeletal Remodeling and Endothelial Barrier. J. Biol. Chem. 2015, 290, 4097–4109. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, M.; Foldes, G. Takes Two: Endothelial-Perivascular Cell Cross-Talk in Vascular Development and Disease. Front. Cardiovasc. Med. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Ma, Y.; Du, J.; Gu, L.; Zheng, L.; Zhang, X. Effect of melatonin on EGF- and VEGF-induced monolayer permeability of HUVECs. Am. J. Physiol. Circ. Physiol. 2019, 316, H1178–H1191. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Rahimi, N. Defenders and Challengers of Endothelial Barrier Function. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Argaw, A.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.; Mahase, S.; Dutta, D.; Seto, J.; Kramer, E.; et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. Mult. Scler. J. 2013, 19, 134. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, J.I.; Shields, D.J.; Barillas, S.G.; Acevedo, L.M.; Murphy, E.; Huang, J.; Scheppke, L.; Stockmann, C.; Johnson, R.S.; Angle, N.; et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 2008, 456, 806–813. [Google Scholar] [CrossRef]
- Lin, C.-I.; Chen, C.-N.; Huang, M.-T.; Lee, S.-J.; Lin, C.-H.; Chang, C.-C.; Lee, H. Lysophosphatidic acid upregulates vascular endothelial growth factor-C and tube formation in human endothelial cells through LPA(1/3), COX-2, and NF-kappa B activation- and EGFR transactivation-dependent mechanisms. Cell. Signal. 2008, 20, 1804–1814. [Google Scholar] [CrossRef]
- Simmons, S.; Erfinanda, L.; Bartz, C.; Kuebler, W.M. Novel mechanisms regulating endothelial barrier function in the pulmonary microcirculation. J. Physiol. 2019, 597, 997–1021. [Google Scholar] [CrossRef]
- Scheppke, L.; Murphy, E.A.; Zarpellon, A.; Hofmann, J.J.; Merkulova, A.; Shields, D.J.; Weis, S.M.; Byzova, T.V.; Ruggeri, Z.M.; Iruela-Arispe, M.L.; et al. Notch promotes vascular maturation by inducing integrin-mediated smooth muscle cell adhesion to the endothelial basement membrane. Blood 2012, 119, 2149–2158. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, C.; Zozulya, A.; Wegener, J.; Galla, H.-J. The impact of glia-derived extracellular matrices on the barrier function of cerebral endothelial cells: An in vitro study. Exp. Cell Res. 2007, 313, 1318–1325. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Perrot, C.Y.; Herrera, J.L.; Fournier-Goss, A.E.; Komatsu, M. Prostaglandin E2 breaks down pericyte-endothelial cell interaction via EP1 and EP4-dependent downregulation of pericyte N-cadherin, connexin-43, and R-Ras. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Li, M.W.M.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Connexin 43 is critical to maintain the homeostasis of the blood-testis barrier via its effects on tight junction reassembly. Proc. Natl. Acad. Sci. USA 2010, 107, 17998–18003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasawa, K.; Chiba, H.; Fujita, H.; Kojima, T.; Saito, T.; Endo, T.; Sawada, N. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J. Cell. Physiol. 2006, 208, 123–132. [Google Scholar] [CrossRef]
- Weiss, A.; Attisano, L. The TGFbeta Superfamily Signaling Pathway. Wiley Interdiscip. Rev. Dev. Boil. 2013, 2, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Ota, M.; Rifkin, D.B. Matrix control of transforming growth factor-beta function. J. Biochem. 2012, 152, 321–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef]
- Engelhardt, S.; Al-Ahmad, A.J.; Gassmann, M.; Ogunshola, O.O. Hypoxia Selectively Disrupts Brain Microvascular Endothelial Tight Junction Complexes through a Hypoxia-Inducible Factor-1 (HIF-1) Dependent Mechanism. J. Cell. Physiol. 2014, 229, 1096–1105. [Google Scholar] [CrossRef]
- Liu, J.; Jin, X.; Liu, K.J.; Liu, W. Matrix Metalloproteinase-2-Mediated Occludin Degradation and Caveolin-1-Mediated Claudin-5 Redistribution Contribute to Blood-Brain Barrier Damage in Early Ischemic Stroke Stage. J. Neurosci. 2012, 32, 3044–3057. [Google Scholar] [CrossRef]
- Sladojevic, N.; Stamatovic, S.M.; Johnson, A.M.; Choi, J.; Hu, A.; Dithmer, S.; Blasig, I.E.; Keep, R.F.; Andjelkovic, A.V. Claudin-1-Dependent Destabilization of the Blood-Brain Barrier in Chronic Stroke. J. Neurosci. 2019, 39, 743–757. [Google Scholar] [CrossRef] [Green Version]
- Kale, G.; Naren, A.P.; Sheth, P.; Rao, R.K. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, ZO-3. Biochem. Biophys. Res. Commun. 2003, 302, 324–329. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hui, Y.N.; Guo, B.; Ma, J.X. Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia and hypoxia involving angiopoietin-1 signal way. Eye 2007, 21, 1501–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worzfeld, T.; Schwaninger, M. Apicobasal polarity of brain endothelial cells. J. Cereb. Blood Flow Metab. 2016, 36, 340–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragoni, S.; Turowski, P. Polarised VEGFA Signalling at Vascular Blood-Neural Barriers. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, L.B.; Burkhart, A.; Moos, T. A Triple Culture Model of the Blood-Brain Barrier Using Porcine Brain Endothelial cells, Astrocytes and Pericytes. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Bryan, B.A.; D’Arnore, P.A. Pericyte isolation and use in endothelial/pericyte coculture models. Mehods Enzym. 2008, 443, 315–331. [Google Scholar] [CrossRef]
- Kulczar, C.; Lubin, K.E.; Lefebvre, S.; Miller, D.W.; Knipp, G.T. Development of a direct contact astrocyte-human cerebral microvessel endothelial cells blood-brain barrier coculture model. J. Pharm. Pharmacol. 2017, 69, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.M.; van Dijk, C.G.M.; Maringanti, R.; Chrifi, I.; Kramann, R.; Verhaar, M.C.; Duncker, D.J.; Mokry, M.; Cheng, C. Transcriptome analysis reveals microvascular endothelial cell-dependent pericyte differentiation. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]







| Pathway | Overlap | Adj. p-Value |
|---|---|---|
| TGF-beta regulation of extracellular matrix | 40/565 | 9.37E-23 |
| Interleukin-1 regulation of extracellular matrix | 16/120 | 2.98E-12 |
| Gastrin pathway | 9/44 | 4.41E-08 |
| TNF-alpha effects on cytokine activity, cell motility, and apoptosis | 12/135 | 5.55E-07 |
| Interleukin-5 regulation of apoptosis | 12/144 | 9.32E-07 |
| Interleukin-2 signaling pathway | 26/847 | 2.30E-06 |
| Immune system signaling by interferons, interleukins, prolactin, and growth hormones | 15/280 | 3.02E-06 |
| Fos-related antigen (FRA) pathway | 7/37 | 3.93E-06 |
| Oncostatin M | 14/311 | 6.16E-05 |
| Interferon alpha/beta signaling | 7/64 | 1.57E-04 |
| Interferon signaling | 10/168 | 2.09E-04 |
| Fibroblast growth factor 1 | 5/26 | 2.75E-04 |
| T cell receptor regulation of apoptosis | 18/603 | 3.63E-04 |
| Thymic stromal lymphopoietin (TSLP) pathway | 7/90 | 0.00112 |
| Receptor of advanced glycation end products (RAGE) pathway | 6/60 | 0.00107 |
| Cytokine-cytokine receptor interaction | 11/265 | 0.00138 |
| Hypertrophy pathway | 4/20 | 0.00182 |
| Activator protein 1 (AP-1) transcription factor network | 6/70 | 0.00219 |
| Epidermal growth factor receptor 1 (EGFR1) pathway | 8/152 | 0.00336 |
| Cytosolic tRNA aminoacylation | 4/24 | 0.00330 |
| Type II interferon signaling (interferon-gamma) | 5/50 | 0.00430 |
| Ataxia-telangiectasia mutated (ATM)-dependent DNA damage response | 6/82 | 0.00441 |
| TNF-like weak inducer of apoptosis (TWEAK) regulation of gene expression | 4/27 | 0.00465 |
| Follicle-stimulating hormone (FSH) regulation of apoptosis | 10/263 | 0.00475 |
| Folate metabolism | 5/63 | 0.0110 |
| Gene | Gene Description | log2 FC (co- vs. Monoculture) | FDR p-Value |
|---|---|---|---|
| Soluble mediators | |||
| CXCL8 | Chemokine (C-X-C motif) ligand 8 | −3.7 | 1.68E-07 |
| CXCL5 | Chemokine (C-X-C motif) ligand 5 | −2.2 | 0.0009 |
| CXCL6 | Chemokine (C-X-C motif) ligand 6 | −1.9 | 0.036 |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 | −1.5 | 0.0045 |
| IL1B | Interleukin 1 beta | −2.4 | 0.0003 |
| IL1A | Interleukin 1 alpha | −1.4 | 0.0021 |
| IL32 | Interleukin-32 | −1.7 | 0.0002 |
| VEGFC | Vascular endothelial growth factor C | −1.3 | 0.0016 |
| TGFB2 | Transforming growth factor beta 2 | 1.1 | 0.016 |
| BMP4 | Bone morphogenetic protein 4 | 1.5 | 0.0048 |
| BMP6 | Bone morphogenetic protein 6 | 1.4 | 0.0021 |
| Junctional Proteins | |||
| CLDN1 | Claudin-1 | −2.0 | 4.69E-05 |
| OCCL | Occludin | −1.1 | 0.034 |
| ECM Proteins | |||
| ADAMTS6 | ADAM metallopeptidase with thrombospondin type 1 motif 6 | −1.3 | 0.0008 |
| LAMB3 | Laminin beta 3 | −1.9 | 0.0008 |
| SERPINE1 | Serpin peptidase inhibitor, calde E (nexin, plasminogen activator inhibitor, type 1) | −1.6 | 0.0012 |
| PLAU | Plasminogen activator, urokinase | −1.5 | 0.0019 |
| LTBP1 | Latent transforming growth factor beta binding protein 1 | −1.1 | 0.0022 |
| EFEMP1 | EGF containing fibulin-like ECM protein1 | 0.86 | 0.0125 |
| SERPINA3 | Serpin peptidase inhibitor, calde A (alpha-1 antiproteinase, antitrypsin) | 1.6 | 0.0252 |
| BGN | Biglycan | 1.5 | 0.0348 |
| Cell surface proteins | |||
| JAG1 | Jagged 1 | −1.2 | 0.020 |
| LPAR1 | Lysophosphatidic acid receptor 1 | −1.3 | 0.0009 |
| LEPR | Leptin receptor | 1.1 | 0.0092 |
| Protein | Array Coordinates | Protein Regulation | mRNA Regulation |
|---|---|---|---|
| CXCL1 | D1 | −55% | down (ns) |
| CXCL5 | C2 | −34% | down |
| CXCL8 | E4 | −25% | down |
| CXCL10 | F10 | −61% | down |
| CXCL11 | F11 | −59% | nr |
| CXCL12 | H12 | −31% | up (ns) |
| IL-1-a | D8 | −64% | down |
| IL-1-b | D9 | −34% | down |
| IL-1-Ra | D10 | −50% | nr |
| IL-1-R4 | I3 | +83% | up |
| IL-3 | D12 | −100% | nr |
| IL-18-BP | E12 | −100% | nr |
| PDGF-AB/-BB | H4 | +31% | nr |
| HGF | D3 | +164% | nr |
| MIC-1 | C10 | −47% | nr |
| CSF-2 | C11 | −46% | nr |
| CSF-3 | C9 | −44% | nr |
| Kallikrein 3 | F12 | −53% | nr |
| PECAM1 (CD31) | J4 | +14% | nr |
| SERPIN E1 | I1 | +16% | down |
| Pentraxin 3 | H5 | −27% | down |
| EGF | B10 | −81% | nr |
| Endoglin | C3 | +10% | up (ns) |
| PLAUR | I10 | −11% | down (ns) |
| DPPIV | B9 | −17% | nr |
| ICAM-1 | D4 | +16% | nr |
| MIF | G7 | +20% | nr |
| Emmprin | B11 | +7% | nr |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurmann, L.; Okoniewski, M.; Ogunshola, O.O.; Leeners, B.; Imthurn, B.; Dubey, R.K. Transcryptomic Analysis of Human Brain-Microvascular Endothelial Response to -Pericytes: Cell Orientation Defines Barrier Function. Cells 2021, 10, 963. https://doi.org/10.3390/cells10040963
Kurmann L, Okoniewski M, Ogunshola OO, Leeners B, Imthurn B, Dubey RK. Transcryptomic Analysis of Human Brain-Microvascular Endothelial Response to -Pericytes: Cell Orientation Defines Barrier Function. Cells. 2021; 10(4):963. https://doi.org/10.3390/cells10040963
Chicago/Turabian StyleKurmann, Lisa, Michal Okoniewski, Omolara O. Ogunshola, Brigitte Leeners, Bruno Imthurn, and Raghvendra K. Dubey. 2021. "Transcryptomic Analysis of Human Brain-Microvascular Endothelial Response to -Pericytes: Cell Orientation Defines Barrier Function" Cells 10, no. 4: 963. https://doi.org/10.3390/cells10040963