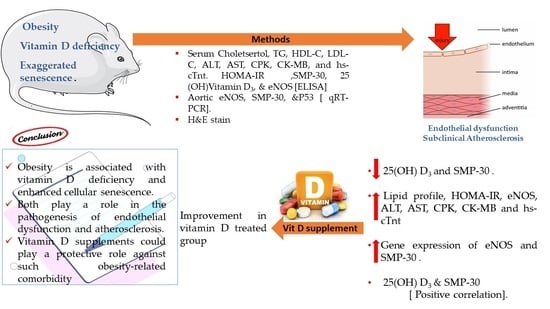
Cellular Senescence and Vitamin D Deficiency Play a Role in the Pathogenesis of Obesity-Associated Subclinical Atherosclerosis: Study of the Potential Protective Role of Vitamin D Supplementation
Abstract
:1. Introduction
2. Methods
2.1. Animals and Experimental Protocol
2.2. Anthropometric Measurement
2.3. Sampling and Biochemical Investigations
2.4. Molecular Study of Gene Expression of eNOS, SMP-30, and P53
2.5. Histological and Morphometric Study
2.6. Statistical Analysis
3. Results
Histological Results
4. Discussion
Limitation of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Approval
References
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Ferrucci, L. Sarcopenic obesity: Definition, cause, and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar]
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. In Obesity and Lipotoxicity; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–17. [Google Scholar]
- Lodish, M.B.; Trivellin, G.; Stratakis, C.A. Current Opinion in Endocrinology, Diabetes, and Obesity “Pituitary gigantism: Update on Molecular Biology and Management”. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 72. [Google Scholar] [CrossRef] [Green Version]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia, and mortality—A review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence for faces of senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Regulski, M.J. Cellular Senescence: What, Why, and How. Wounds Compend. Clin. Res. Pract. 2017, 29, 168–174. [Google Scholar]
- Burton, D.G.; Faragher, R.G. Obesity and type-2 diabetes as inducers of premature cellular senescence and aging. Biogerontology 2018, 19, 447–459. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.S.; Pilkerton, C.S.; Shrader, C.D.; Frisbee, S.J. Subclinical atherosclerosis, cardiovascular health, and disease risk: Is there a case for the Cardiovascular Health Index in the primary prevention population? BMC Public Health 2018, 18, 429. [Google Scholar] [CrossRef]
- Ortega, F.B.; Lee, D.C.; Katzmarzyk, P.T.; Ruiz, J.R.; Sui, X.; Church, T.S.; Blair, S.N. The intriguing metabolically healthy but obese phenotype: Cardiovascular prognosis and role of fitness. Eur. Heart J. 2013, 34, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tousoulis, D.; Oikonomou, E.; Maniatis, K.; Kioufis, S.; Zaromitidou, M.; Kokkou, E.; Mazaris, S.; Konsola, T.; Stefanadis, C. Vitamin D serum levels are associated with cardiovascular outcome in coronary artery disease. Int. J. Cardiol. 2013, 168, 4445–4447. [Google Scholar] [CrossRef]
- Lai, H.; Fishman, E.K.; Gerstenblith, G.; Brinker, J.A.; Tong, W.; Bhatia, S.; Detrick, B.; Lai, S. Vitamin D deficiency is associated with significant coronary stenoses in asymptomatic African American chronic cocaine users. Int. J. Cardiol. 2012, 158, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Alvira, J.M.; Fuster, V.; Dorado, B.; Soberón, N.; Flores, I.; Gallardo, M.; Pocock, S.; Blasco, M.A.; Andrés, V. Short telomere load, telomere length, and subclinical atherosclerosis: The PESA study. J. Am. Coll. Cardiol. 2016, 67, 2467–2476. [Google Scholar] [CrossRef] [Green Version]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli Filho, J.L.V.B. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Farhangi, M.A.; Mesgari-Abbasi, M.; Nameni, G.; Hajiluian, G.; Shahabi, P. The effects of vitamin D administration on brain inflammatory markers in high-fat diet-induced obese rats. BMC Neurosci. 2017, 18, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, D.; Hosker, J.; Rudenski, A.; Naylor, B.; Treacher, D.; Turner, R. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Gu, X.; Yu, M.; Zi, Y.; Yu, H.; Wang, Y.; Xie, Y.; Xiang, L. Effects of ginsenoside Rb1 on oxidative stress injury in rat spinal cords by regulating the eNOS/Nrf2/HO-1 signaling pathway. Exp. Ther. Med. 2018, 16, 1079–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arafat, E.A.; Ghoneim, F.M.; Khalaf, H.A.; Elsamanoudy, A.Z. Anti-senescence role of coenzyme Q10 and 17 β-estradiol on submandibular gland of ovariectomized rats: Histological, immunohistological and molecular studies. Int. J. Clin. Exp. Pathol. 2016, 9, 10853–10870. [Google Scholar]
- Tokumoto, M.; Fujiwara, Y.; Shimada, A.; Hasegawa, T.; Seko, Y.; Nagase, H.; Satoh, M. Cadmium toxicity is caused by accumulation of p53 through the down-regulation of Ube2d family genes in vitro and in vivo. J. Toxicol. Sci. 2011, 36, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Stavenuiter, A.W.; Arcidiacono, M.V.; Ferrantelli, E.; Keuning, E.D.; Vila Cuenca, M.; ter Wee, P.M.; Beelen, R.H.; Vervloet, M.G.; Dusso, A.S. A novel rat model of vitamin D deficiency: Safe and rapid induction of vitamin D and calcitriol deficiency without hyperparathyroidism. BioMed Res. Int. 2015, 2015, 604275. [Google Scholar] [CrossRef] [Green Version]
- De Pergola, G.; Martino, T.; Zupo, R.; Caccavo, D.; Pecorella, C.; Paradiso, S.; Silvestris, F.; Triggiani, V. 25 Hydroxyvitamin D Levels are Negatively and Independently Associated with Fat Mass in a Cohort of Healthy Overweight and Obese Subjects. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 838–844. [Google Scholar] [CrossRef]
- Munasinghe, L.L.; Yuan, Y.; Willows, N.D.; Faught, E.L.; Ekwaru, J.P.; Veugelers, P.J. Vitamin D deficiency and sufficiency among Canadian children residing at high latitude following the revision of the RDA of vitamin D intake in 2010. Br. J. Nutr. 2017, 117, 457–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakharova, I.N.; Mal’tsev, S.V.; Borovik, T.E.; Yatsyk, G.V.; Malyavskaya, S.I.; Vakhlova, I.V.; Shumatova, T.A.; Romantsova, Y.B.; Romanyuk, F.P.; Klimov, L.Y.; et al. Vitamin D insufficiency in children of tender years in Russia: The results of a multi-center cohort study Rodnichok (2013–2014). Curr. Pediatrics 2014, 13, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Ardawi, M.-S.; Sibiany, A.; Bakhsh, T.; Qari, M.; Maimani, A. High prevalence of vitamin D deficiency among healthy Saudi Arabian men: Relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporos. Int. 2012, 23, 675–686. [Google Scholar] [CrossRef]
- Elsamanoudy, A.Z.; Hassanien, M.; Khayyat, M.M.; Ardawi, M.-S.M. Study of the Possible Effect of Obesity on Vitamin D Level among a Sample of Adult Men in the Western Region of Kingdom of Saudi Arabia. Int. J. Biochem. Res. Rev. 2016, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.C.; Zhu, Y.M.; Ping, G.U.O.; Zheng, C.H.E.N.; Xie, F.Z.; Liu, X.L.; Shan, H.E. Serum 25 (OH) D and lipid levels in Chinese obese and normal-weight males before and after oral vitamin D supplementation. Biomed. Environ. Sci. 2013, 26, 801–807. [Google Scholar] [PubMed]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Carrelli, A.; Bucovsky, M.; Horst, R.; Cremers, S.; Zhang, C.; Bessler, M.; Schrope, B.; Evanko, J.; Blanco, J.; Silverberg, S.J.; et al. Vitamin D storage in adipose tissue of obese and normal weight women. J. Bone Miner. Res. 2017, 32, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Gangloff, A.; Bergeron, J.; Lemieux, I.; Despres, J.P. Changes in circulating vitamin D levels with loss of adipose tissue. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 464–470. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Milic, S.; Wensveen, T.T.; Grgic, I.; Jakopcic, I.; Stimac, D.; Wensveen, F.; Orlic, L. Nonalcoholic fatty liver disease—A multisystem disease? World J. Gastroenterol. 2016, 22, 9488–9505. [Google Scholar] [CrossRef]
- Vranic, L.; Mikolasevic, I.; Milic, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef] [Green Version]
- Wamberg, L.; Pedersen, S.B.; Rejnmark, L.; Richelsen, B. Causes of vitamin D deficiency and effect of vitamin D supplementation on metabolic complications in obesity: A review. Curr. Obes. Rep. 2015, 4, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, S.M.; Park, H.S.; Choi, K.M.; Cho, G.J.; Ko, B.J.; Kim, J.H. Serum 25-hydroxyvitamin D levels, obesity and the metabolic syndrome among Korean children. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 785–791. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Harris, R.A.; Hatahet, Z.; Chou, K.-M. Ablation of XP-V gene causes adipose tissue senescence and metabolic abnormalities. Proc. Natl. Acad. Sci. USA 2015, 112, E4556–E4564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pusceddu, I.; Farrell, C.-J.L.; Di Pierro, A.M.; Jani, E.; Herrmann, W.; Herrmann, M. The role of telomeres and vitamin D in cellular aging and age-related diseases. Clin. Chem. Lab. Med. 2015, 53, 1661–1678. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. Vitamin D deficiency accelerates ageing and age-related diseases: A novel hypothesis. J. Physiol. 2017, 595, 6825–6836. [Google Scholar] [CrossRef]
- Carrillo-Vega, M.F.; García-Peña, C.; Gutiérrez-Robledo, L.M.; Pérez-Zepeda, M.U. Vitamin D deficiency in older adults and its associated factors: A cross-sectional analysis of the Mexican Health and Aging Study. Arch. Osteoporos. 2017, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Scaini, G.; Rezin, G.T.; Carvalho, A.F.; Streck, E.L.; Berk, M.; Quevedo, J. Mitochondrial dysfunction in bipolar disorder: Evidence, pathophysiology and translational implications. Neurosci. Biobehav. Rev. 2016, 68, 694–713. [Google Scholar] [CrossRef]
- De Pergola, G.; Nitti, A.; Bartolomeo, N.; Gesuita, A.; Giagulli, V.A.; Triggiani, V.; Guastamacchia, E.; Silvestris, F. Possible role of hyperinsulinemia and insulin resistance in lower vitamin D levels in overweight and obese patients. Biomed. Res. Int. 2013, 2013, 921348. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.M.; Shi, M.; Cheng, A.; Gao, Y.; Chen, G.; Song, X.; So, R.W.L.; Zhang, J.; Herrup, K. Age-related hyperinsulinemia leads to insulin resistance in neurons and cell-cycle-induced senescence. Nat. Neurosci. 2019, 22, 1806–1819. [Google Scholar] [CrossRef] [PubMed]
- Csige, I.; Ujvárosy, D.; Szabó, Z.; Lőrincz, I.; Paragh, G.; Harangi, M.; Somodi, S. The impact of obesity on the cardiovascular system. J. Diabetes Res. 2018, 2018, 3407306. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, T.; Dohi, Y.; Takagi, Y.; Yoshikane, N.; Ito, M.; Suzuki, K.; Nagami, T.; Iwase, M.; Seo, Y.; Ohte, N. Relationships of Obesity-Related Indices and Metabolic Syndrome with Subclinical Atherosclerosis in Middle-Aged Untreated Japanese Workers. J. Atheroscler. Thromb. 2020, 27, 342–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, J.T.; Nambi, V.; De Lemos, J.A.; Chambless, L.E.; Virani, S.S.; Boerwinkle, E.; Hoogeveen, R.C.; Liu, X.; Astor, B.C.; Mosley, T.H.; et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011, 123, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Ndumele, C.E.; Coresh, J.; Lazo, M.; Hoogeveen, R.C.; Blumenthal, R.S.; Folsom, A.R.; Selvin, E.; Ballantyne, C.M.; Nambi, V. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail. 2014, 2, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Altin, C.; Söylev, G.Ö.; Tekindal, M.A.; Agildere, M. Assessment of Subclinical Atherosclerosis in Vitamin D Deficiency. Ultrasound Q. 2019, 35, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Yiu, Y.F.; Yiu, K.H.; Siu, C.W.; Chan, Y.H.; Li, S.W.; Wong, L.Y.; Lee, S.W.; Tam, S.; Wong, E.W.; Lau, C.P.; et al. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis 2013, 227, 140–146. [Google Scholar] [CrossRef]
- Satilmis, S.; Celik, O.; Biyik, I.; Ozturk, D.; Celik, K.A.; Akın, F.; Ayca, B.; Yalcin, B.; Dagdelen, S. Association between serum vitamin D levels and subclinical coronary atherosclerosis and plaque burden/composition in young adult population. Bosn. J. Basic Med. Sci. 2015, 15, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehnder, D.; Bland, R.; Chana, R.S.; Wheeler, D.C.; Howie, A.J.; Williams, M.C.; Stewart, P.M.; Hewison, M. Synthesis of 1, 25-dihydroxyvitamin D3 by human endothelial cells is regulated by inflammatory cytokines: A novel autocrine determinant of vascular cell adhesion. J. Am. Soc. Nephrol. 2002, 13, 621–629. [Google Scholar] [PubMed]
- Al Mheid, I.; Patel, R.; Murrow, J.; Morris, A.; Rahman, A.; Fike, L.; Kavtaradze, N.; Uphoff, I.; Hooper, C. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J. Am. Coll. Cardiol. 2011, 58, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Weng, S.; Felton, S.K.; Bhandare, S.; Riek, A.; Butler, B.; Proctor, B.M.; Petty, M.; Chen, Z.; Schechtman, K.B.; et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 2009, 120, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuumi, G.; Shimizu, I.; Yoshida, Y.; Minamino, T. Vascular senescence in cardiovascular and metabolic diseases. Front. Cardiovasc. Med. 2018, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Imga, N.N.; Karci, A.C.; Oztas, D.; Berker, D.; Guler, S. Effects of vitamin D supplementation on insulin resistance and dyslipidemia in overweight and obese premenopausal women. Arch. Med. Sci. AMS 2019, 15, 598. [Google Scholar] [CrossRef]
- Chen, L.; Yang, R.; Qiao, W.; Zhang, W.; Chen, J.; Mao, L.; Goltzman, D.; Miao, D. 1, 25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell 2019, 18, e12951. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc, S.; Cimpean, A.M.; Mitu, F.; Heredea, R.; Iurciuc, M. Vascular aging and subclinical atherosclerosis: Why such a “never ending” and challenging story in cardiology? Clin. Interv. Aging 2017, 12, 1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faridi, K.F.; Lupton, J.R.; Martin, S.S.; Banach, M.; Quispe, R.; Kulkarni, K.; Jones, S.R.; Michos, E.D. Vitamin D deficiency and non-lipid biomarkers of cardiovascular risk. Arch. Med. Sci. 2017, 13, 732. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Chen, Y.D.; Tian, F.; Yang, N.; Cheng, L.Q.; Hu, S.Y.; Wang, J.; Yang, J.J.; Wang, S.F.; Gu, X.F. Effects of liraglutide on reperfusion injury in patients with ST-segment-elevation myocardial infarction. Circ. Cardiovasc. Imaging 2016, 9, e005146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golforoush, P.; Yellon, D.M.; Davidson, S.M. Mouse models of atherosclerosis and their suitability for the study of myocardial infarction. Basic Res. Cardiol. 2020, 115, 73. [Google Scholar] [CrossRef]


| Negative Control n = 10 | Positive Control (Vit D) n = 10 | Obese Group n = 24 | Obese+ Vitamin-D Supplement n = 13 | ANOVA p Value | |
|---|---|---|---|---|---|
| Weight (gm) | 194.80 ± 5.71 | 199.70 ± 8.48 c,d | 336.33 ± 24.35 a,b,d | 308.61 ± 22.39 a,b,c | <0.001 * |
| Height (cm) | 21.26 ± 1.40 | 21.22 ± 0.83 | 21.56 ± 1.00 | 21.38 ± 0.70 | 0.767 |
| BMI (gm/cm2) | 0.43 ± 0.06 | 0.44 ± 0.03 c,d | 0.74 ± 0.07 a,b | 0.72 ± 0.07 a,b | <0.001 * |
| Intima/Media Thickness (IMT) (μm) | 59.5 ± 2.9 | 60.4 ± 3.1 | 98.6 ± 8.1 a,b | 65.3 ± 4.2 c | <0.001 * |
| Insulin (uIU/mL) | 2.21 ± 0.13 | 1.93 ± 0.07 a,c,d | 4.80 ± 0.29 a,b,d | 2.30 ± 0.17 b,c | <0.001 * |
| Glucose (mg/dL) | 113.22 ± 16.00 | 113.45 ± 16.91 c | 206.13 ± 117.20 a,b,d | 102.30 ± 18.67 c | <0.001 * |
| HOMA-IR | 0.60 ± 0.10 | 0.55 ± 0.08 c | 2.45 ± 1.38 a,b,d | 0.58 ± 0.10 c | <0.001 * |
| TG (mg/dL) | 133.11 ± 7.00 | 118.15 ± 11.27 c,d | 269.41 ± 27.05 a,b,d | 166.37 ± 6.57 a,c,d | <0.001 * |
| CHOL (mg/dL) | 182.40 ± 4.41 | 112.87 ± 6.75 a,c,d | 268.11 ± 10.79 a,b,d | 216.54 ± 17.87 a,b,c | <0.001 * |
| LDL-C (mg/dL) | 130.93 ± 8.84 | 109.85 ± 6.61 a,c,d | 241.07 ± 5.35 a,b,d | 153.19 ± 3.70 a,b,c | <0.001 * |
| HDL-C (mg/dL) | 52.99 ± 3.66 | 81.58 ± 3.79 a,c,d | 39.11 ± 1.67 a.b.d | 45.18 ± 3.10 a,b,c | <0.001 * |
| eNOS (ng/mL) | 0.37 ± 0.15 | 0.56 ± 0.25 c | 1.54 ± 0.55 a,b,d | 0.82 ± 0.65 a,c | <0.001 * |
| hs-cTnt (pg/mL) | 1.72 ± 0.36 | 2.96 ± 1.83 c | 6.11 ± 1.83 a,b,d | 2.34 ± 1.45 c | <0.001 * |
| CK_MB (U/L) | 191.66 ± 28.23 | 92.80 ± 7.62 a,c,d | 683.49 ± 140.71 a,b,d | 367.84 ± 51.84 a,b,c | <0.001 * |
| CK (U/L) | 499.40 ± 123.60 | 275.00 ± 56.23 c,d | 3365.41 ± 707.24 a,b,d | 774.07 ± 74.98 b,c | <0.001 * |
| ALT (U/L) | 40.50 ± 3.62 | 19.80 ± 5.05 a,c,d | 83.87 ± 6.25 a,c,d | 56.38 ± 6.18 a,b,d | <0.001 * |
| AST (U/L) | 141.20 ± 13.90 | 53.10 ± 8.46 a,c,d | 250.33 ± 16.00 a,b,d | 185.30 ± 9.54 a,b,c | <0.001 * |
| Ca (mg/dL) | 9.84 ± 0.13 | 9.91 ± 0.12 | 9.79 ± 0.16 | 9.87 ± 0.09 | 0.61 |
| P (mg/dL) | 3.79 ± 0.11 | 3.82 ± 0.14 | 3.70 ± 0.19 | 3.81 ± 0.09 | 0.72 |
| 25 Hydroxycholecalciferol (nmol/L) | 5.95 ± 1.08 | 110.00 ± 8.89 a,c,d | 4.44 ± 1.25 b,d | 75.49 ± 6.72 a,b,c | <0.001 * |
| SMP-30 (pg/mL) | 133.10 ± 19.20 | 170.50 ± 29.80 a,c,d | 58.50 ± 22.06 a,b | 66.30 ± 18.76 a,b | <0.001 * |
| Negative Control n = 10 | Positive Control (Vit D) n = 10 | Obese Group n = 24 | Obese + Vitamin D Supplement n = 13 | ANOVA p Value | |
|---|---|---|---|---|---|
| eNOS gene expression (2−∆∆CT) | 1 ± 0.02 | 1.74 ± 0.21 a,c,d | 2.16 ± 0.25 a,b,d | 1.38 ± 0.19 a,b,c | <0.001 * |
| SMP-30 gene expression (2−∆∆CT) | 1 ± 0.03 | 1.14 ± 0.03 c | 0.55 ± 0.15 a,b,d | 1.15 ± 0.23 c | <0.001 * |
| P53 gene expression (2−∆∆CT) | 1 ± 0.02 | 1.74 ± 0.18 a,c,d | 2.26 ± 0.27 a,b,d | 1.48 ± 0.23 a,b,c | <0.001 * |
| All the Studied Population | Negative Control (Vit D) n = 10 | Positive Control n = 10 | Obese Group n = 24 | Obese +Vitamin-D Supplement n = 13 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | r | p-value | |
| Weight (gm) | −0.38 | 0.00 * | 0.22 | 0.54 | 0.18 | 0.61 | 0.06 | 0.79 | −0.07 | 0.82 |
| Height (cm) | −0.12 | 0.87 | −0.44 | 0.21 | −0.53 | 0.11 | −0.23 | 0.28 | −0.31 | 0.31 |
| BMI (gm/cm2) | −0.21 | 0.03 * | 0.53 | 0.12 | 0.46 | 0.18 | 0.05 | 0.81 | 0.34 | 0.25 |
| Insulin u/IU/mL) | −0.58 | 0.00 * | 0.05 | 0.89 | 0.48 | 0.16 | 0.04 | 0.85 | −0.29 | 0.34 |
| Glucose (mg/dL) | −0.25 | 0.05 * | 0.90 | 0.00 | 0.49 | 0.15 | 0.25 | 0.23 | 0.60 | 0.03 |
| Homa-IR | −0.38 | 0.00 * | 0.39 | 0.27 | 0.37 | 0.29 | 0.23 | 0.27 | 0.41 | 0.17 |
| LDL-C (mg/dL) | −0.65 | 0.00 * | 0.73 | 0.02 * | −0.14 | 0.61 | −0.29 | 0.17 | 0.32 | 0.28 |
| HDL-C (mg/dL) | 0.91 | 0.00 * | −0.21 | 0.41 | 0.56 | 0.09 | −0.02 | 0.91 | 0.21 | 0.49 |
| Chol (mg/dL) | −0.80 | 0.00 * | 0.15 | 0.67 | 0.32 | 0.37 | −0.02 | 0.94 | 0.59 | 0.04 * |
| TG (mg/dL) | −0.51 | 0.00 * | −0.63 | 0.05 * | −0.02 | 0.95 | 0.27 | 0.20 | 0.16 | 0.60 |
| hs-cTnt (pg/mL) | −0.27 | 0.04 * | −0.56 | 0.09 * | 0.42 | 0.23 | 0.03 | 0.88 | −0.10 | 0.73 |
| CK (U/I) | −0.56 | 0.00 * | 0.47 | 0.17 | −0.02 | 0.94 | 0.11 | 0.61 | −0.04 | 0.91 |
| CK-MB (U/I) | −0.62 | 0.00 * | 0.76 | 0.01 * | −0.21 | 0.55 | 0.26 | 0.23 | −0.29 | 0.34 |
| AST (U/I) | −0.82 | 0.00 * | −0.01 | 0.79 | −0.61 | 0.05 * | −0.12 | 0.59 | −0.09 | 0.76 |
| ALT (U/I) | −0.75 | 0.00 * | −0.30 | 0.39 | −0.13 | 0.72 | −0.12 | 0.57 | −0.14 | 0.66 |
| eNOS (ng/mL) | −0.34 | 0.10 | 0.05 | 0.81 | −0.00 | 0.91 | 0.04 | 0.84 | −0.36 | 0.23 |
| SMP-30 (pg/mL) | 0.65 | <0.00 * | 0.29 | 0.42 | 0.16 | 0.65 | −0.03 | 0.88 | −0.17 | 0.58 |
| All the Studied Population | Negative Control (Vit D) n = 10 | Positive Control n = 10 | Obese Group n = 24 | Obese + Vitamin-D Supplement n = 13 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | r | p-value | |
| Weight (gm) | −0.80 | <0.00 * | 0.41 | 0.24 | −0.22 | 0.55 | 0.01 | 0.65 | 0.61 | 0.03 * |
| Height (cm) | −0.19 | 0.16 | −0.29 | 0.41 | −0.33 | 0.35 | −0.01 | 0.98 | −0.34 | 0.26 |
| BMI (gm/cm2) | −0.81 | <0.00 * | 0.31 | 0.38 | 0.10 | 0.78 | −0.37 | 0.078 | 0.04 | 0.81 |
| Insulin u/IU/mL) | −0.64 | 0.00 | −0.13 | 0.72 | 0.24 | 0.51 | 0.19 | 0.39 | −0.22 | 0.47 |
| Glucose (mg/dL) | −0.24 | 0.04 | 0.06 | 0.86 | −0.09 | 0.80 | 0.13 | 0.56 | −0.00 | 0.99 |
| Homa-IR | −0.31 | 0.00 | 0.11 | 0.77 | −0.23 | 0.53 | 0.03 | 0.95 | −0.33 | 0.35 |
| LDL-C (mg/dL) | −0.73 | 0.00 | −0.12 | 0.73 | 0.26 | 0.46 | 0.30 | 0.15 | −0.04 | 0.90 |
| HDL-C (mg/dL) | 0.84 | 0.00 | −0.67 | 0.04 | 0.50 | 0.14 | 0.22 | 0.31 | −0.01 | 0.98 |
| CHOL (mg/dL) | −0.82 | 0.00 | 0.12 | 0.75 | −0.30 | 0.39 | 0.31 | 0.14 | 0.−01 | 0.98 |
| TG (mg/dL) | −0.69 | 0.00 | −0.36 | 0.31 | 0.50 | 0.14 | 0.13 | 0.53 | 0.11 | 0.72 |
| hs-cTnt (pg/mL) | −0.41 | 0.00 | −0.24 | 0.51 | 0.39 | 0.27 | −0.11 | 0.36 | −0.27 | 0.38 |
| CK (U/I) | −0.64 | 0.00 | 0.41 | 0.24 | 0.66 | 0.04 | −0.18 | 0.41 | 0.48 | 0.01 |
| CK-MB (U/I) | −0.73 | <0.00 * | −0.02 | 0.96 | 0.27 | 0.44 | 0.19 | 0.39 | 0.34 | 0.26 |
| AST (U/I) | −0.85 | <0.00 * | 0.04 | 0.91 | −0.39 | 0.26 | −0.12 | 0.58 | −0.40 | 0.17 |
| ALT (U/I) | −0.83 | <0.00 * | −0.49 | 0.15 | −0.17 | 0.64 | −0.16 | 0.45 | −0.23 | 0.45 |
| eNOS (ng/mL) | −0.47 | <0.00 * | 0.55 | 0.01 | 0.33 | 0.35 | −0.26 | 0.23 | 0.47 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bima, A.I.; Mahdi, A.S.; Al Fayez, F.F.; Khawaja, T.M.; Abo El-Khair, S.M.; Elsamanoudy, A.Z. Cellular Senescence and Vitamin D Deficiency Play a Role in the Pathogenesis of Obesity-Associated Subclinical Atherosclerosis: Study of the Potential Protective Role of Vitamin D Supplementation. Cells 2021, 10, 920. https://doi.org/10.3390/cells10040920
Bima AI, Mahdi AS, Al Fayez FF, Khawaja TM, Abo El-Khair SM, Elsamanoudy AZ. Cellular Senescence and Vitamin D Deficiency Play a Role in the Pathogenesis of Obesity-Associated Subclinical Atherosclerosis: Study of the Potential Protective Role of Vitamin D Supplementation. Cells. 2021; 10(4):920. https://doi.org/10.3390/cells10040920
Chicago/Turabian StyleBima, Abdulhadi I., Abdullah S. Mahdi, Fayza F. Al Fayez, Taghreed M. Khawaja, Salwa M. Abo El-Khair, and Ayman Z. Elsamanoudy. 2021. "Cellular Senescence and Vitamin D Deficiency Play a Role in the Pathogenesis of Obesity-Associated Subclinical Atherosclerosis: Study of the Potential Protective Role of Vitamin D Supplementation" Cells 10, no. 4: 920. https://doi.org/10.3390/cells10040920