Adsorption of Cd to TiO2-NPs Forms Low Genotoxic Aggregates in Zebrafish Cells
Abstract
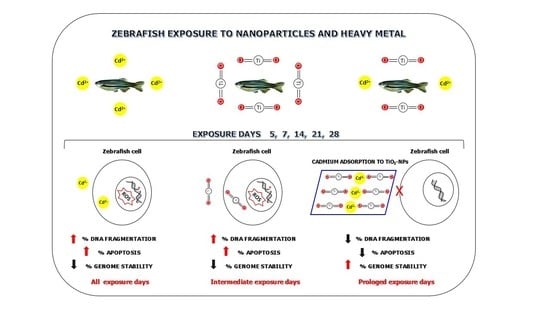
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Specimens Preparation
2.3. Specimens Sacrifice
2.4. Characterization and Analytical Determinations
2.5. Comet Assay
2.6. Diffusion Assay
2.7. RAPD-PCR Technique and %GTS Calculation
2.8. Statistical Analysis
3. Results
3.1. Characterization and Analytical Determinations
3.2. Comet Assay
3.3. Diffusion Assay
3.4. RAPD-PCR Technique
3.5. Genomic Template Stability (GTS, °/°)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frenzilli, G.; Bernardeschi, M.; Guidi, P.; Scarcelli, V.; Lucchesi, P.; Marsili, L.; Fossi, M.C.; Brunelli, A.; Pojana, G.; Marcomini, A.; et al. Effects of in vitro exposure to titanium dioxide on DNA integrity of bottlenose dolphin (Tursiops truncatus) fibroblasts and leukocytes. Mar. Environ. Res. 2014, 100, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Nigro, M.; Bernardeschi, M.; Costagliola, D.; Della Torre, C.; Frenzilli, G.; Guidi, P.; Lucchesi, P.; Mottola, F.; Santonastaso, M.; Scarcelli, V.; et al. n-TiO2 and CdCl2 co-exposure to titanium dioxide nanoparticles and cadmium: Genomic, DNA and chromosomal damage evaluation in the marine fish European sea bass (Dicentrarchus labrax). Aquat. Toxicol. 2015, 168, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, C.; Buonocore, F.; Frenzilli, G.; Corsolini, S.; Brunelli, A.; Guidi, P.; Kočan, A.; Mariottini, M.; Mottola, F.; Nigro, M.; et al. Influence of titanium dioxide nanoparticles on 2,3,7,8-tetrachlorodibenzo-p-dioxin bioconcentration and toxicity in the marine fish European sea bass (Dicentrarchus labrax). Environ. Pollut. 2015, 196, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: A Review. Biol. Trace Element Res. 2016, 172, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, Q.; Jiang, L.; Yu, Z.; Jiang, D.; Yin, D. Combined effects of titanium dioxide and humic acid on the bioaccumulation of cadmium in Zebrafish. Environ. Pollut. 2011, 159, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Schaumann, G.E.; Philippe, A.; Bundschuh, M.; Metreveli, G.; Klitzke, S.; Rakcheev, D.; Grün, A.Y.; Kumahor, S.; Kühn, M.; Baumann, T.; et al. Understanding the fate and biological effects of Ag- and TiO2-nanoparticles in the environment: The quest for advanced analytics and interdisciplinary concepts. Sci. Total Environ. 2015, 535, 3–19. [Google Scholar] [CrossRef]
- Rocco, L.; Santonastaso, M.; Mottola, F.; Costagliola, D.; Suero, T.; Pacifico, S.; Stingo, V. Genotoxicity assessment of TiO2 nanoparticles in the teleost Danio rerio. Ecotoxicol. Environ. Saf. 2015, 113, 223–230. [Google Scholar] [CrossRef]
- Fako, V.E.; Furgeson, D.Y. Zebrafish as a correlative and predictive model for assessing biomaterial nanotoxicity. Adv. Drug Deliv. Rev. 2009, 61, 478–486. [Google Scholar] [CrossRef]
- Brundo, M.V.; Epecoraro, R.; Emarino, F.; Esalvaggio, A.; Etibullo, D.; Esaccone, S.; Ebramanti, V.; Buccheri, M.A.; Eimpellizzeri, G.; Escuderi, V.; et al. Toxicity Evaluation of New Engineered Nanomaterials in Zebrafish. Front. Physiol. 2016, 7, 130. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, Z.; Zhu, X. Toxic Effects of TiO2 NPs on Zebrafish. Int. J. Environ. Res. Public Health 2019, 16, 523. [Google Scholar] [CrossRef] [Green Version]
- Dubey, A.; Goswami, M.; Yadav, K.; Chaudhary, D. Oxidative Stress and Nano-Toxicity Induced by TiO2 and ZnO on WAG Cell Line. PLoS ONE 2015, 10, e0127493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, C.; Coppola, F.; Monteiro, R.; Russo, T.; Polese, G.; Lourenço, M.A.; Silva, M.R.F.; Ferreira, P.; Soares, A.M.; Freitas, R.; et al. Biochemical and histopathological impacts of rutile and anatase (TiO2 forms) in Mytilus galloprovincialis. Sci. Total Environ. 2020, 719, 134886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottola, F.; Iovine, C.; Santonastaso, M.; Romeo, M.L.; Pacifico, S.; Cobellis, L.; Rocco, L. NPs-TiO2 and lincomycin coexposure induces DNA damage in cultured human amniotic cells. Nanomaterials 2019, 9, 1511. [Google Scholar]
- Fan, W.H.; Cui, M.M.; Shi, Z.W.; Tan, C.; Yang, X.P. Enhanced oxidative stress and physiological damage in Daphnia magna by copper in the presence of nano-TiO2. J. Nanomater. 2012, 2012, 398720. [Google Scholar] [CrossRef] [Green Version]
- Turner, A. Cadmium pigments in consumer products and their health risks. Sci. Total Environ. 2019, 657, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Rocco, L.; Santonastaso, M.; Nigro, M.; Mottola, F.; Costagliola, D.; Bernardeschi, M.; Guidi, P.; Lucchesi, P.; Scarcelli, V.; Corsi, I.; et al. Genomic and chromosomal damage in the marine mussel Mytilus galloprovincialis: Effects of the combined exposure to titanium dioxide nanoparticles and cadmium chloride. Mar. Environ. Res. 2015, 111, 144–148. [Google Scholar] [CrossRef]
- Chang, K.-C.; Hsu, C.-C.; Liu, S.-H.; Su, C.-C.; Yen, C.-C.; Lee, M.-J.; Chen, K.-L.; Ho, T.-J.; Hung, D.-Z.; Wu, C.-C.; et al. Cadmium Induces Apoptosis in Pancreatic β-Cells through a Mitochondria-Dependent Pathway: The Role of Oxidative Stress-Mediated c-Jun N-Terminal Kinase Activation. PLoS ONE 2013, 8, e54374. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-K.; Bork, U.; Gholamrezaei, F.; Thévenod, F. Cd2+-induced cytochrome c release in apoptotic proximal tubule cells: Role of mitochondrial permeability transition pore and Ca2+ uniporter. Am. J. Physiol. Physiol. 2005, 288, F27–F39. [Google Scholar] [CrossRef]
- Casalino, E.; Calzaretti, G.; Sblano, C.; Landriscina, V.; Tecce, M.F.; Landriscina, C. Antioxidant effect of hydroxytyrosol (DPE) and Mn2+ in liver of cadmium-intoxicated rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 625–632. [Google Scholar] [CrossRef]
- Wang, L.; Yan, B.; Liu, N.; Li, Y.; Wang, Q. Effects of cadmium on glutathione synthesis in hepatopancreas of freshwater crab, Sinopotamon yangtsekiense. Chemosphere 2008, 74, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Trenner, A.; Sartori, A.A. Harnessing DNA Double-Strand Break Repair for Cancer Treatment. Front. Oncol. 2019, 9, 1388. [Google Scholar] [CrossRef] [PubMed]
- Naasz, S.; Altenburger, R.; Kühnel, D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total Environ. 2018, 635, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wang, M.; Gong, S.; Tian, B. Impacts of Sediment Organic Matter Content and pH on Ecotoxicity of Coexposure of TiO2 Nanoparticles and Cadmium to Freshwater Snails Bellamya aeruginosa. Arch. Environ. Contam. Toxicol. 2016, 72, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pei, J.; Tang, X.; Guo, X. Effects of surfactants on the combined toxicity of TiO2 nanoparticles and cadmium to Escherichia coli. J. Environ. Sci. 2018, 74, 126–133. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, H.; Zhang, Z.; Niu, Q.; Chen, Y.; Crittenden, J. Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere 2007, 67, 160–166. [Google Scholar] [CrossRef]
- Jönsson, M.E.; Brunström, B.; Brandt, I. The zebrafish gill model: Induction of CYP1A, EROD and PAH adduct formation. Aquat. Toxicol. 2009, 91, 62–70. [Google Scholar] [CrossRef]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Singh, N.P. A Simple Method for Accurate Estimation of Apoptotic Cells. Exp. Cell Res. 2000, 256, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Kašuba, V.; Rozgaj, R.; Milić, M.; Želježić, D.; Kopjar, N.; Pizent, A.; Kljaković-Gašpić, Z.; Jazbec, A. Evaluation of genotoxic effects of lead in pottery-glaze workers using micronucleus assay, alkaline comet assay and DNA diffusion assay. Int. Arch. Occup. Environ. Health 2011, 85, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Cantafora, E.; Giorgi, F.S.; Frenzilli, G.; Scarcelli, V.; Busceti, C.L.; Nigro, M.; Bernardeschi, M.; Fornai, F. Region-specific DNA alterations in focally induced seizures. J. Neural Transm. 2014, 121, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Rocco, L.; Frenzilli, G.; Fusco, D.; Peluso, C.; Stingo, V. Evaluation of zebrafish DNA integrity after exposure to pharmacological agents present in aquatic environments. Ecotoxicol. Environ. Saf. 2010, 73, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Rocco, L.; Valentino, I.V.; Scapigliati, G.; Stingo, V. RAPD-PCR analysis for molecular characterization and genotoxic studies of a new marine fish cell line derived from Dicentrarchus labrax. Cytotechnology 2013, 66, 383–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Yang, Y.; Zhou, Q.; Xie, L.; Li, P.; Sun, T. Impact assessment of cadmium contamination on rice (Oryza sativa L.) seedlings at molecular and population levels using multiple biomarkers. Chemosphere 2007, 67, 1155–1163. [Google Scholar] [CrossRef]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini-Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef]
- Czajka, M.; Sawicki, K.; Sikorska, K.; Popek, S.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of titanium dioxide nanoparticles in central nervous system. Toxicol. Vitr. 2015, 29, 1042–1052. [Google Scholar] [CrossRef]
- Handl, J.; Čapek, J.; Majtnerová, P.; Petira, F.; Hauschke, M.; Roušarová, E.; Roušar, T. Transient Increase In Cellular Dehydrogenase Activity After Cadmium Treatment Precedes Enhanced Production Of Reactive Oxygen Species In Human Proximal Tubular Kidney Cells. Physiol. Res. 2019, 68, 481–490. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2017, 119, 157–184. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Colacurci, N.; Iovine, C.; Pacifico, S.; Cammarota, M.; Cesaroni, F.; Rocco, L. In Vitro Genotoxic Effects of Titanium Dioxide Nanoparticles (N-Tio2) in Human Sperm Cells. Mol. Reprod. Dev. 2019, 86, 1369–1377. [Google Scholar] [CrossRef]
- Gaubin, Y.; Vaissade, F.; Croute, F.; Beau, B.; Soleilhavoup, J.-P.; Murat, J. Implication of free radicals and glutathione in the mechanism of cadmium-induced expression of stress proteins in the A549 human lung cell-line. Biochim. Biophys. Acta (BBA)-Bioenerg. 2000, 1495, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Balestri, M.; Ceccarini, A.; Forino, L.M.C.; Zelko, I.; Martinka, M.; Lux, A.; Castiglione, M.R. Cadmium uptake, localization and stress-induced morphogenic response in the fern Pteris vittata. Planta 2014, 239, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, M.L.; Grassi, G.; Leaver, M.J.; Corsi, I. Combination effects of nano-TiO2 and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on biotransformation gene expression in the liver of European sea bass Dicentrarchus labrax. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 176–177, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Ciacci, C.; Balbi, T. Interactive effects of nanoparticles with other contaminants in aquatic organisms: Friend or foe? Mar. Environ. Res. 2015, 111, 128–134. [Google Scholar] [CrossRef]
- Frenzilli, G.; Nigro, M.; Lyons, B.P. The Comet assay for the evaluation of genotoxic impact in aquatic environments. Mutat. Res. Mutat. Res. 2009, 681, 80–92. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Adsorption Mechanisms of Organic Chemicals on Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Rocco, L.; Mottola, F.; Santonastaso, M.; Saputo, V.; Cusano, E.; Costagliola, D.; Suero, T.; Pacifico, S.; Stingo, V. Anti-genotoxic ability of α-tocopherol and Anthocyanin to counteract fish DNA damage induced by musk xylene. Ecotoxicology 2015, 24, 2026–2035. [Google Scholar] [CrossRef]
- Mottola, F.; Scudiero, N.; Iovine, C.; Santonastaso, M.; Rocco, L. Protective activity of ellagic acid in counteract oxidative stress damage in zebrafish embryonic development. Ecotoxicol. Environ. Saf. 2020, 197, 110642. [Google Scholar] [CrossRef]
- Bassing, C.H.; Alt, F.W. The cellular response to general and programmed DNA double strand breaks. DNA Repair 2004, 3, 781–796. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Iovine, C.; Cesaroni, F.; Colacurci, N.; Rocco, L. In Vitro Effects of Titanium Dioxide Nanoparticles (TiO2NPs) on Cadmium Chloride (CdCl2) Genotoxicity in Human Sperm Cells. Nanomaterials 2020, 10, 1118. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yan, L.; Chan, T.; Jing, C. Molecular Insights into Ternary Surface Complexation of Arsenite and Cadmium on TiO2. Environ. Sci. Technol. 2015, 49, 5973–5979. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Flora, G.; Yadav, M.; Tiwari, A. A review with recent advancements on bioremediation-based abolition of heavy metals. Environ. Sci. Process. Impacts 2014, 16, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Hosu, O.; Tertis, M.; Cristea, C. Hosu Implication of Magnetic Nanoparticles in Cancer Detection, Screening and Treatment. Magnetochemistry 2019, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.C.N.; Lo, I.M. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef]
- Quiroga-Flores, R.; Noshad, A.; Wallenberg, R.; Önnby, L. Adsorption Of Cadmium By A High-Capacity Adsorbent Composed Of Silicate-Titanate Nanotubes Embedded In Hydrogel Chitosan Beads. Environ. Technol. 2015, 41, 3043–3054. [Google Scholar] [CrossRef]
- Caponetti, V.; Trzcinski, J.W.; Cantelli, A.; Tavano, R.; Papini, E.; Mancin, F.; Montalti, M. Self-Assembled Biocompatible Fluorescent Nanoparticles for Bioimaging. Front. Chem. 2019, 7, 168. [Google Scholar] [CrossRef]






| Treatment | Exposure Days | Gained Bands | Lost Bands a |
|---|---|---|---|
| Cd (1 mg/L) | 5 7 14 21 28 | 650, 1000 690 350, 650, 690, 780, 850 - 180, 300, 420, 850 | 200, 220, 290, 320 200, 230, 290, 320, 550, 800 290, 320 220, 250, 320, 600 290, 400, 600, 800 |
| TiO2-NPs (10 μg/L) | 5 7 14 21 28 | 100, 690 100, 690 100, 420, 690 100, 420 380, 420, 480 | 220 220 220, 250, 300, 320 320, 500, 550 - |
| TiO2-NPs (10 μg/L) + Cd (1 mg/L) | 5 7 14 21 28 | 100, 420, 480, 690 300, 420, 480 180, 850 - - | - 200, 220 290, 320 550 600 |
| Benzene (10 µL/mL) | 5 7 14 21 28 | 180, 650, 690 - 100, 580, 650, 690 150, 180, 580, 690 150, 420, 430, 850 | 200, 220, 290, 320 200, 230, 290, 320, 550, 800 290, 320, 450, 550 250, 400, 600, 800 200, 400, 550, 800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottola, F.; Santonastaso, M.; Iovine, C.; Feola, V.; Pacifico, S.; Rocco, L. Adsorption of Cd to TiO2-NPs Forms Low Genotoxic Aggregates in Zebrafish Cells. Cells 2021, 10, 310. https://doi.org/10.3390/cells10020310
Mottola F, Santonastaso M, Iovine C, Feola V, Pacifico S, Rocco L. Adsorption of Cd to TiO2-NPs Forms Low Genotoxic Aggregates in Zebrafish Cells. Cells. 2021; 10(2):310. https://doi.org/10.3390/cells10020310
Chicago/Turabian StyleMottola, Filomena, Marianna Santonastaso, Concetta Iovine, Veronica Feola, Severina Pacifico, and Lucia Rocco. 2021. "Adsorption of Cd to TiO2-NPs Forms Low Genotoxic Aggregates in Zebrafish Cells" Cells 10, no. 2: 310. https://doi.org/10.3390/cells10020310