Role of Aldynoglia Cells in Neuroinflammatory and Neuroimmune Responses after Spinal Cord Injury
Abstract
:1. Introduction
2. Characteristics of Aldynoglia Cells in Brain
3. Morphological and Functional Characteristics of Tanycytes and Pituicytes
4. Olfactory Ensheathing Cells
5. Müller Cells
6. Bergmann Glial Cells
7. Pineal Gland Cells
8. Aldynoglia Cells in Spinal Cord
9. Secondary Cell Death in Spinal Cord after Injury
- Acute phase, spanning 0 to 2 days after the injury,
- Subacute phase, 3 days to weeks after the injury,
- Chronic phase, 7 weeks to years after the injury,
10. Innate Immune Response in Spinal Cord Injury
11. The Adaptive Immune Response after SCI
12. Phagocytic Immune Cells in Acute and Sub-Acute Phases of SCI
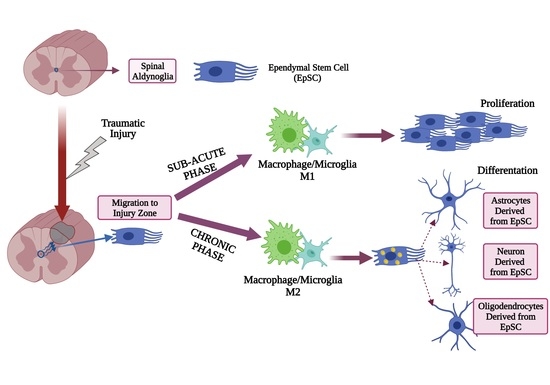
13. The Transition from M1 to M2 Phenotype for Microglia/Macrophage in Sub-Acute and Chronic Phases after SCI
14. M1 Macrophage/Microglia Promotes Proliferation of Aldynoglia Cells in Sub-Acute Phase after SCI
15. M2 Macrophage/Microglia Induce Aldynoglial Cell Differentiation in Chronic Phase of SCI
16. Chronic Phase of Traumatic Spinal Cord Injury: The Activity of Aldynoglia
17. Effect of Aldynoglia Cells on Regeneration after SCI
18. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, Y.S.; Choi, J.; Yoon, B.-E. Neuron-Glia Interactions in Neurodevelopmental Disorders. Cells 2020, 9, 2176. [Google Scholar] [CrossRef]
- Simons, M.; Nave, K.-A. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb. Perspect. Biol. 2016, 8, a020479. [Google Scholar] [CrossRef]
- Doncel-Pérez, E.; Nieto-Sampedro, M. Aldynoglia cells and modulation of RhoGTpase activity as useful tools for spinal cord injury repair. Neural Regen. Res. 2016, 11, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Murtaza, M.; Velasquez, J.T.; Todorovic, M.; Rayfield, A.; Ekberg, J.; Barton, M.; St John, J. Olfactory Ensheathing Cells for Spinal Cord Injury: Sniffing Out the Issues. Cell Transplant. 2018, 27, 879–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudiño-Cabrera, G.; Nieto-Sampedro, M. Schwann-like macroglia in adult rat brain. Glia 2000, 30, 49–63. [Google Scholar] [CrossRef]
- Ortuno-Sahagun, D.; Rojas-Mayorquín, A.E.; Camins, A.; Pallàs, M. Embryonic Neural Stem Cell Differentiation to Aldynoglia Induced by Olfactory Bulb Ensheathing Cell-Conditioned Medium. In Embryonic Stem Cells: The Hormonal Regulation of Pluripotency and Embryogenesis; InTech Open: Rijeka, Croatia, 2011. [Google Scholar]
- Liu, Y.; Rao, M.S. Glial progenitors in the CNS and possible lineage relationships among them. Biol. Cell 2004, 96, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Doncel-Pérez, E.; Caballero-Chacón, S.; Nieto-Sampedro, M. Neurosphere cell differentiation to aldynoglia promoted by olfactory ensheathing cell conditioned medium. Glia 2009, 57, 1393–1409. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S. Advances in understanding of structural reorganization in the hypothalamic neurosecretory system. Front. Endocrinol. (Lausanne) 2017, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Wittkowski, W. Tanycytes and pituicytes: Morphological and functional aspects of neuroglial interaction. Microsc. Res. Tech. 1998, 41, 29–42. [Google Scholar] [CrossRef]
- Reshamwala, R.; Shah, M.; St John, J.; Ekberg, J. Survival and Integration of Transplanted Olfactory Ensheathing Cells are Crucial for Spinal Cord Injury Repair: Insights from the Last 10 Years of Animal Model Studies. Cell Transplant. 2019, 28, 132S–159S. [Google Scholar] [CrossRef] [PubMed]
- Lebrun-Julien, F.; Morquette, B.; Douillette, A.; Saragovi, H.U.; Di Polo, A. Inhibition of p75NTR in glia potentiates TrkA-mediated survival of injured retinal ganglion cells. Mol. Cell. Neurosci. 2009, 40, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Hippert, C.; Graca, A.B.; Basche, M.; Kalargyrou, A.A.; Georgiadis, A.; Ribeiro, J.; Matsuyama, A.; Aghaizu, N.; Bainbridge, J.W.; Smith, A.J.; et al. RNAi-mediated suppression of vimentin or glial fibrillary acidic protein prevents the establishment of Müller glial cell hypertrophy in progressive retinal degeneration. Glia 2021, 69, 2272–2290. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.W.; Li, J.Y.H. The Molecular Pathway Regulating Bergmann Glia and Folia Generation in the Cerebellum. Cerebellum 2018, 17, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Welsh, M.G. S-Antigen and glial fibrillary acidic protein immunoreactivity in the in situ pineal gland of hamster and gerbil and in pineal grafts: Developmental expression of pinealocyte and glial markers. Am. J. Anat. 1991, 192, 510–522. [Google Scholar] [CrossRef]
- Jouvet, A.; Derrington, E.; Pialat, J.; Lapras, C.; Fèvre-Montange, M.; Besançon, R.; Belin, M.F.; Saint-Pierre, G. Structural and ultrastructural characteristics of human pineal gland, and pineal parenchymal tumors. Acta Neuropathol. 1994, 88, 334–348. [Google Scholar] [CrossRef]
- Sanin, V.; Heeß, C.; Kretzschmar, H.A.; Schüller, U. Recruitment of neural precursor cells from circumventricular organs of patients with cerebral ischaemia. Neuropathol. Appl. Neurobiol. 2013, 39, 510–518. [Google Scholar] [CrossRef]
- Hatton, J. Pituicytes, glia and control of terminal secretion. J. Exp. Biol. 1988, 139, 67–79. [Google Scholar] [CrossRef]
- Gudiño-Cabrera, G.; Nieto-Sampedro, M. Estrogen receptor immunoreactivity in schwann-like brain macroglia. J. Neurobiol. 1999, 40, 458–470. [Google Scholar] [CrossRef]
- Daniel, G. Múller glia cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 23, 1–7. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. New functions of müller cells. Glia 2013, 61, 651–678. [Google Scholar] [CrossRef]
- Haiwei, X.; Yang, Y.; Xiaotong, T.; Meina, Z.; Fucheng, L.; Pie, X.; Baoke, H.; Yan, X.; Xiaohang, B.; Xiaotang, F. Bergmann Glia Function in Granulle Cel Migration During Cerebellum Development. Mol. Neurobiol. 2013, 47, 833–844. [Google Scholar]
- Buffo, A.; Rossi, F. Origin, lineage and function of cerebellar glia. Prog. Neurobiol. 2013, 109, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.P.I.; Noctor, S.C.; Muñoz, E.M. Cellular basis of pineal gland development: Emerging role of microglia as phenotype regulator. PLoS ONE 2016, 11, e0167063. [Google Scholar] [CrossRef] [Green Version]
- Bukreeva, I.; Junemann, O.; Cedola, A.; Palermo, F.; Maugeri, L.; Begani Provinciali, G.; Pieroni, N.; Sanna, A.; Otlyga, D.A.; Buzmakov, A.; et al. Investigation of the human pineal gland 3D organization by X-ray phase contrast tomography. J. Struct. Biol. 2020, 212, 107659. [Google Scholar] [CrossRef]
- Moore, S.A. The Spinal Ependymal Layer in Health and Disease. Vet. Pathol. 2016, 53, 746–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisén, J.; Johansson, C.B.; Török, C.; Risling, M.; Lendahl, U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J. Cell Biol. 1995, 131, 453–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawsey, T.; Duflou, J.; Weickert, C.S.; Gorrie, C.A. Nestin-Positive Ependymal Cells Are Increased in the Human Spinal Cord after Traumatic Central Nervous System Injury. J. Neurotrauma 2015, 32, 1393–1402. [Google Scholar] [CrossRef]
- McDonough, A.; Martínez-Cerdeño, V. Endogenous Proliferation after Spinal Cord Injury in Animal Models. Stem Cells Int. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Becker, C.G.; Becker, T.; Hugnot, J.-P. The spinal ependymal zone as a source of endogenous repair cells across vertebrates. Prog. Neurobiol. 2018, 170, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Bruni, J.E.; Del Bigio, M.R.; Clattenburg, R.E. Ependyma: Normal and pathological. A review of the literature. Brain Res. Rev. 1985, 9, 1–19. [Google Scholar] [CrossRef]
- Leclerc, A.; Matveeff, L.; Emery, E. Syringomyelia and hydromyelia: Current understanding and neurosurgical management. Rev. Neurol. (Paris) 2021, 177, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Chacón, S.C.; Nieto-sampedro, M. Pathophysiology of spinal cord injury. A review. Med. Vet. 2005, 36, 75–86. [Google Scholar]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. (Wars) 2011, 71, 281–299. [Google Scholar]
- Anjum, A.; Yazid, M.D.; Daud, M.F.; Idris, J.; Hwei Ng, A.M.; Naicker, A.S.; Rashidah Ismail, O.H.; Kumar, R.K.A.; Lokanathan, Y. Spinal cord injury: Pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Riegger, T.; Conrad, S.; Liu, K.; Schluesener, H.J.; Adibzahdeh, M.; Schwab, J.M. Spinal cord injury-induced immune depression syndrome (SCI-IDS). Eur. J. Neurosci. 2007, 25, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Brommer, B.; Engel, O.; Kopp, M.A.; Watzlawick, R.; Müller, S.; Prüss, H.; Chen, Y.; DeVivo, M.J.; Finkenstaedt, F.W.; Dirnagl, U.; et al. Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain 2016, 139, 692–707. [Google Scholar] [CrossRef] [Green Version]
- Prüss, H.; Tedeschi, A.; Thiriot, A.; Lynch, L.; Loughhead, S.M.; Stutte, S.; Mazo, I.B.; Kopp, M.A.; Brommer, B.; Blex, C.; et al. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat. Neurosci. 2017, 20, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.; Aguilar-Cevallos, J.; Silva-Garcia, R.; Ibarra, A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediat. Inflamm. 2016, 2016, 9476020. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of secondary spinal cord injury. Front. Cell. Neurosci. 2016, 10, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Ankeny, D.P.; Popovich, P.G. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience 2009, 158, 1112–1121. [Google Scholar] [CrossRef] [Green Version]
- David, S.; Zarruk, J.G.; Ghasemlou, N. Inflammatory Pathways in Spinal Cord Injury. Int. Rev. Neurobiol. 2012, 106, 127–152. [Google Scholar] [PubMed]
- Zhou, X.; He, X.J.; Ren, Y. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen. Res. 2014, 9, 1787–1795. [Google Scholar] [PubMed]
- Akhmetzyanova, E.; Kletenkov, K.; Mukhamedshina, Y.; Rizvanov, A. Different Approaches to Modulation of Microglia Phenotypes After Spinal Cord Injury. Front. Syst. Neurosci. 2019, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- David, S.; Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Jalessi, M.; Jameie, S.B.; Khanmohammadi, M.; Bagher, Z.; Namjoo, Z.; Davachi, S.M. More attention on glial cells to have better recovery after spinal cord injury. Biochem. Biophys. Rep. 2021, 25, 100905. [Google Scholar]
- Ma, Y.; Deng, M.; Zhao, X.Q.; Liu, M. Alternatively polarized macrophages regulate the growth and differentiation of ependymal stem cells through the SIRT2 pathway. Exp. Neurobiol. 2020, 29, 150–163. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, M.; Liu, M. Effect of Differently Polarized Macrophages on Proliferation and Differentiation of Ependymal Cells from Adult Spinal Cord. J. Neurotrauma 2019, 36, 2337–2347. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]
- Ren, Y.; Ao, Y.; O’Shea, T.M.; Burda, J.E.; Bernstein, A.M.; Brumm, A.J.; Muthusamy, N.; Ghashghaei, H.T.; Carmichael, S.T.; Cheng, L.; et al. Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dependent on direct ependymal injury. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Meletis, K.; Barnabé-Heider, F.; Carlén, M.; Evergren, E.; Tomilin, N.; Shupliakov, O.; Frisén, J. Spinal Cord Injury Reveals Multilineage Differentiation of Ependymal Cells. PLoS Biol. 2008, 6, e182. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Buzoianu-Anguiano, V.; Rivera-Osorio, J.; Orozco-Suárez, S.; Vega-García, A.; García-Vences, E.; Sánchez-Torres, S.; Jiménez-Estrada, I.; Guizar-Sahagún, G.; Mondragon-Caso, J.; Fernández-Valverde, F.; et al. Single vs. Combined Therapeutic Approaches in Rats with Chronic Spinal Cord Injury. Front. Neurol. 2020, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Cho, Y.S.; Park, J.M.; Na, Y.G.; Kim, K.H. Stem Cell Therapy for Neurogenic Bladder After Spinal Cord Injury: Clinically Possible? Int. Neurourol. J. 2020, 24, S3–S10. [Google Scholar] [CrossRef]
- Zhu, Y.; Uezono, N.; Yasui, T.; Nakashima, K. Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Dev. Dyn. 2018, 247, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, G.D.; Kepler, C.K.; Vaccaro, A.R. The Use of Cell Transplantation in Spinal Cord Injuries. J. Am. Acad. Orthop. Surg. 2016, 24, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Marichal, N.; Reali, C.; Rehermann, M.I.; Trujillo-Cenóz, O.; Russo, R.E. Progenitors in the ependyma of the spinal cord: A potential resource for self-repair after injury. Adv. Exp. Med. Biol. 2017, 1015, 241–264. [Google Scholar] [CrossRef]
- Ruzicka, J.; Machova-Urdzikova, L.; Gillick, J.; Amemori, T.; Romanyuk, N.; Karova, K.; Zaviskova, K.; Dubisova, J.; Kubinova, S.; Murali, R.; et al. A Comparative Study of Three Different Types of Stem Cells for Treatment of Rat Spinal Cord Injury. Cell Transplant. 2017, 26, 585–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Zheng, J.; Bian, G.; Liu, L.; Xue, Q.; Liu, F.; Yu, C.; Zhang, H.; Song, B.; Chung, S.K.; et al. Polarized Macrophages Have Distinct Roles in the Differentiation and Migration of Embryonic Spinal-cord-derived Neural Stem Cells After Grafting to Injured Sites of Spinal Cord. Mol. Ther. 2015, 23, 1077–1091. [Google Scholar] [CrossRef] [Green Version]
- Roet, K.C.D.; Verhaagen, J. Understanding the neural repair-promoting properties of olfactory ensheathing cells. Exp. Neurol. 2014, 261, 594–609. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, I.; Fernández-Mayoralas, A.; Moreno-Lillo, S.; Sánchez-Sierra, M.; Nieto-Sampedro, M.; Doncel-Pérez, E. Inhibition of glial proliferation, promotion of axonal growth and myelin production by synthetic glycolipid: A new approach for spinal cord injury treatment. Restor. Neurol. Neurosci. 2015, 33. [Google Scholar] [CrossRef]
- Becker, C.G.; Becker, T. Neuronal Regeneration from Ependymo-Radial Glial Cells: Cook, Little Pot, Cook! Dev. Cell 2015, 32, 516–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, M. Restoring neuro-immune circuitry after brain and spinal cord injuries. Int. Immunol. 2021, 33, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Hayano, Y.; Nakagawa, H.; Yamashita, T. Intraspinal rewiring of the corticospinal tract requires target-derived brain-derived neurotrophic factor and compensates lost function after brain injury. Brain 2012, 135, 1253–1267. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Nie, E.H.; Yin, Y.; Benowitz, L.I.; Tung, S.; Vinters, H.V.; Bahjat, F.R.; Stenzel-Poore, M.P.; Kawaguchi, R.; Coppola, G.; et al. GDF10 is a signal for axonal sprouting and functional recovery after stroke. Nat. Neurosci. 2015, 18, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.J.; Piltti, K.M.; Hooshmand, M.J.; Nishi, R.A.; Cummings, B.J. Preclinical Efficacy Failure of Human CNS-Derived Stem Cells for Use in the Pathway Study of Cervical Spinal Cord Injury. Stem Cell Rep. 2017, 8, 249–263. [Google Scholar] [CrossRef] [PubMed]




| Location In Brain | Cellular Type | Markers | Bibliography |
|---|---|---|---|
| Subventricular zone, third ventricle | Tanycytes | GFAP, p75 NGFR, vimentin, S100β | [9,10] |
| Hypothalamus | Pituicytes | ||
| Olfactory Bulb | Ensheathing olfactory cells | GFAP, p75 NGFR, vimentin, S100β, ERα | [11] |
| Retina | Müller glia cells | GFAP, p75 NGFR, vimentin. | [12,13] |
| Ventricular area of the cerebellum | Bergmann glia cells | GFAP, p75 NGFR, vimentin, S100β, S0X1, SOX2, SOX9. | [14] |
| Pineal gland | Pinealocytes | GFAP, vimentin. | [15,16,17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzoianu-Anguiano, V.; Torres-Llacsa, M.; Doncel-Pérez, E. Role of Aldynoglia Cells in Neuroinflammatory and Neuroimmune Responses after Spinal Cord Injury. Cells 2021, 10, 2783. https://doi.org/10.3390/cells10102783
Buzoianu-Anguiano V, Torres-Llacsa M, Doncel-Pérez E. Role of Aldynoglia Cells in Neuroinflammatory and Neuroimmune Responses after Spinal Cord Injury. Cells. 2021; 10(10):2783. https://doi.org/10.3390/cells10102783
Chicago/Turabian StyleBuzoianu-Anguiano, Vinnitsa, Mabel Torres-Llacsa, and Ernesto Doncel-Pérez. 2021. "Role of Aldynoglia Cells in Neuroinflammatory and Neuroimmune Responses after Spinal Cord Injury" Cells 10, no. 10: 2783. https://doi.org/10.3390/cells10102783