SCFA Treatment Alleviates Pathological Signs of Migraine and Related Intestinal Alterations in a Mouse Model of NTG-Induced Migraine
Abstract
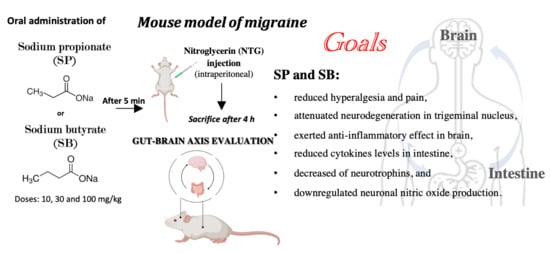
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Migraine Model Induction
Experimental Groups
- -
- Group sham + vehicle (veh): mice received saline;
- -
- Group NTG: mice received NTG (10 mg/kg) intraperitoneally;
- -
- Group NTG + sumatriptan: mice received sumatriptan orally (600 μg/kg) 5 min after NTG (10 mg/kg) intraperitoneally;
- -
- Group NTG + SP 10 mg/kg: mice received SP orally at a dose of 10 mg/kg 5 min after NTG injection;
- -
- Group NTG + SP 30 mg/kg: mice received SP orally at a dose of 30 mg/kg 5 min after NTG injection;
- -
- Group NTG + SP 100 mg/kg: mice received SP orally at a dose of 100 mg/kg 5 min after NTG injection;
- -
- Group NTG + SB 10 mg/kg: mice received SB orally at a dose of 10 mg/kg 5 min after NTG injection;
- -
- Group NTG + SB 30 mg/kg: mice received SB orally at a dose of 30 mg/kg 5 min after NTG injection;
- -
- Group NTG + SB 100 mg/kg: mice received SB orally at a dose of 100 mg/kg 5 min after NTG injection.
2.3. Behavioral Tests
2.3.1. Tail Flick Test
2.3.2. Orofacial Formalin Test
2.3.3. Hot Plate Test
2.3.4. Light/Dark Test
2.3.5. Histological Analysis
2.3.6. Western Blot Analysis of COX2 and iNOS
2.3.7. Immunohistochemical Localization of Tumor Necrosis Factor, Interleukin-1β, and Neuronal Nitric Oxide Synthase in the Intestine
2.3.8. Immunofluorescence Localization of Brain-Derived Nerve Factor and Neurotrophin-3 in the Intestine
2.3.9. ELISA Kit Assay
2.3.10. Real-Time Quantitative PCR Amplification
2.3.11. Statistical Evaluation
3. Results
3.1. SCFA Treatments Reduced NTG-Induced Hyperalgesia and Pain
3.2. NTG-Induced Neurodegeneration in Trigeminal Nucleus Is Attenuated by SCFA Treatments
3.3. The Effects of SCFAs on the Anti-Inflammatory Pathway in NTG-Induced Migraine
3.4. SCFA Treatments Attenuate Intestinal Alterations following NTG Injection
3.5. Increased Cytokine Release Is Reduced by SCFA Treatments following NTG-Induced Migraine in the Intestine
3.6. SCFA Administration Contributes to Decreased Neurotrophin Intestinal Immunoreactivity following NTG-Induced Migraine
3.7. Neuronal Nitric Oxide Production Is Downregulated following SCFA Administration in NTG-Injected Mice
3.8. SCFA Treatments Modulate Proinflammatory Mediators following NTG-Induced Migraine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goadsby, P.J.; Lipton, R.B.; Ferrari, M.D. Migraine—Current understanding and treatment. N. Engl. J. Med. 2002, 346, 257–270. [Google Scholar] [CrossRef] [Green Version]
- Lipton, R.B.; Bigal, M.E.; Diamond, M.; Freitag, F.; Reed, M.L.; Stewart, W.F.; on behalf of the AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007, 68, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, D.S.; Reddy, S. The pathophysiological and pharmacological basis of current drug treatment of migraine headache. Expert Rev. Clin. Pharmacol. 2013, 6, 271–288. [Google Scholar] [CrossRef]
- Sanchez-Del-Rio, M.; Reuter, U.; A Moskowitz, M. New insights into migraine pathophysiology. Curr. Opin. Neurol. 2006, 19, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. Migraine Triggers and Oxidative Stress: A Narrative Review and Synthesis. Headache J. Head Face Pain 2015, 56, 12–35. [Google Scholar] [CrossRef]
- Geyik, S.; Altunısık, E.; Neyal, A.M.; Taysi, S. Oxidative stress and DNA damage in patients with migraine. J. Headache Pain 2016, 17, 10. [Google Scholar] [CrossRef] [Green Version]
- Durham, P.; Papapetropoulos, S. Biomarkers Associated with Migraine and Their Potential Role in Migraine Management. Headache J. Head Face Pain 2013, 53, 1262–1277. [Google Scholar] [CrossRef] [PubMed]
- Dalkara, T.; Nozari, A.; A Moskowitz, M. Migraine aura pathophysiology: The role of blood vessels and microembolisation. Lancet Neurol. 2010, 9, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Arzani, M.; On behalf of the School of Advanced Studies of the European Headache Federation (EHF-SAS); Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M. Gut-brain Axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef] [Green Version]
- Aurora, S.K.; Papapetropoulos, S.; Kori, S.H.; Kedar, A.; Abell, T.L. Gastric stasis in migraineurs: Etiology, characteristics, and clinical and therapeutic implications. Cephalalgia 2013, 33, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Goadsby, P.J.; Sprenger, T. Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol. 2010, 9, 285–298. [Google Scholar] [CrossRef]
- Lanza, M.; Campolo, M.; Casili, G.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Sodium Butyrate Exerts Neuroprotective Effects in Spinal Cord Injury. Mol. Neurobiol. 2018, 56, 3937–3947. [Google Scholar] [CrossRef]
- Filippone, A.; Lanza, M.; Campolo, M.; Casili, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. The Anti-Inflammatory and Antioxidant Effects of Sodium Propionate. Int. J. Mol. Sci. 2020, 21, 3026. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; Reyes-Gavilán, C.G.D.L.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [Green Version]
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balázsi, S.; Hajnády, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 2018, 9, 105. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.M.; Bast, A.; Vanhoutvin, S.A.; Fischer, M.A.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.-J.M. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009, 28, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [Green Version]
- Filippone, A.; Lanza, M.; Campolo, M.; Casili, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Protective effect of sodium propionate in Abeta1-42 -induced neurotoxicity and spinal cord trauma. Neuropharmacology 2020, 166, 107977. [Google Scholar] [CrossRef]
- Inan, M.S.; Rasoulpour, R.J.; Yin, L.; Hubbard, A.K.; Rosenberg, D.W.; Giardina, C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 2000, 118, 724–734. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb. Ecol. Health Dis. 2016, 27, 634. [Google Scholar] [CrossRef]
- Besten, G.D.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Bates, E.A.; Nikai, T.; Brennan, K.C.; Fu, Y.H.; Charles, A.C.; Basbaum, A.I.; Ptacek, L.J.; Ahn, A.H. Sumatriptan alleviates nitroglycer-in-induced mechanical and thermal allodynia in mice. Cephalalgia 2010, 30, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, M.D.; Roon, K.I.; Lipton, R.B.; Goadsby, P.J. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: A meta-analysis of 53 trials. Lancet 2001, 358, 1668–1675. [Google Scholar] [CrossRef]
- Meymandi, M.S.; Keyhanfar, F.; Yazdanpanah, O.; Heravi, G. The Role of NMDARs Ligands on Antinociceptive Effects of Pregabalin in the Tail Flick Test. Anesthesiol. Pain Med. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Sufka, K.J.; Staszko, S.M.; Johnson, A.P.; Davis, M.E.; Davis, R.E.; Smitherman, T.A. Clinically relevant behavioral endpoints in a recurrent nitroglycerin migraine model in rats. J. Headache Pain 2016, 17, 40. [Google Scholar] [CrossRef] [Green Version]
- Raboisson, P.; Dallel, R. The orofacial formalin test. Neurosci. Biobehav. Rev. 2004, 28, 219–226. [Google Scholar] [CrossRef]
- Singh, P.; Kongara, K.; Harding, D.; Ward, N.; Dukkipati, V.S.R.; Johnson, C.; Chambers, P. Comparison of electroencephalo-graphic changes in response to acute electrical and thermal stimuli with the tail flick and hot plate test in rats administered with opiorphin. BMC Neurol. 2018, 18, 43. [Google Scholar] [CrossRef] [Green Version]
- Vuralli, D.; Wattiez, A.-S.; Russo, A.F.; Bolay, H. Behavioral and cognitive animal models in headache research. J. Headache Pain 2019, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Casili, G.; Lanza, M.; Filippone, A.; Campolo, M.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Dimethyl fumarate alleviates the nitroglycerin (NTG)-induced migraine in mice. J. Neuroinflamm. 2020, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Lanza, M.; Paterniti, I.; Filippone, A.; Ardizzone, A.; Casili, G.; Scuderi, S.; Puglisi, C.; Mare, M.; Memeo, L.; et al. PEA-OXA Mitigates Oxaliplatin-Induced Painful Neuropathy through NF-κB/Nrf-2 Axis. Int. J. Mol. Sci. 2021, 22, 3927. [Google Scholar] [CrossRef]
- Calabrese, G.; Ardizzone, A.; Campolo, M.; Conoci, S.; Esposito, E.; Paterniti, I. Beneficial Effect of Tempol, a Membrane-Permeable Radical Scavenger, on Inflammation and Osteoarthritis in In Vitro Models. Biomolecules 2021, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, K.; Artola, A.; Monconduit, L.; Dallel, R.; Luccarini, P. Bilateral descending hypothalamic projections to the spinal trigeminal nucleus caudalis in rats. PLoS ONE 2013, 8, e73022. [Google Scholar] [CrossRef]
- Noseda, R.; Burstein, R. Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013, 154, S44–S53. [Google Scholar] [CrossRef] [Green Version]
- Andrews, C.; McLean, M.; Durum, S.K. Cytokine Tuning of Intestinal Epithelial Function. Front. Immunol. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Meir, M.; Burkard, N.; Ungewiß, H.; Diefenbacher, M.; Flemming, S.; Kannapin, F.; Germer, C.-T.; Schweinlin, M.; Metzger, M.; Waschke, J.; et al. Neurotrophic factor GDNF regulates intestinal barrier function in inflammatory bowel disease. J. Clin. Investig. 2019, 129, 2824–2840. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J. Gastroenterol. 2003, 38, 421–430. [Google Scholar] [CrossRef]
- Koçer, A.; Memisogullari, R.; Domac, F.M.; Ilhan, A.; Kocer, E.; Okuyucu, Ş.; Özdemir, B.; Yüksel, H. IL-6 Levels in Migraine Patients Receiving Topiramate. Pain Pract. 2009, 9, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Duarte, H.; Teixeira, A.L.; Rocha, N.P.; Domingues, R.B. Increased interictal serum levels of CXCL8/IL-8 and CCL3/MIP-1α in migraine. Neurol. Sci. 2015, 36, 203–208. [Google Scholar] [CrossRef]
- Goadsby, P.; Holland, P.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Arulmozhi, D.; Veeranjaneyulu, A.; Bodhankar, S. Migraine: Current concepts and emerging therapies. Vasc. Pharmacol. 2005, 43, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Waeber, C.; Moskowitz, M.A. Migraine as an inflammatory disorder. Neurology 2005, 64 (Suppl. 2), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, S.; Shu, H.; Yanagisawa, L.; Tao, F. Gut Microbiota Dysbiosis Enhances Migraine-Like Pain Via TNFalpha Upreg-ulation. Mol. Neurobiol. 2020, 57, 461–468. [Google Scholar] [CrossRef]
- Li, H.-L.; Lu, L.; Wang, X.-S.; Qin, L.-Y.; Wang, P.; Qiu, S.-P.; Wu, H.; Huang, F.; Zhang, B.-B.; Shi, H.-L.; et al. Alteration of Gut Microbiota and Inflammatory Cytokine/Chemokine Profiles in 5-Fluorouracil Induced Intestinal Mucositis. Front. Cell. Infect. Microbiol. 2017, 7, 455. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Costantini, T.; Kroll, L.; Peterson, C.; Loomis, W.; Eliceiri, B.; Baird, A.; Wolf, P.; Coimbra, R. Traumatic Brain Injury and Intestinal Dysfunction: Uncovering the Neuro-Enteric Axis. J. Neurotrauma 2009, 26, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Chalazonitis, A. Neurotrophin-3 in the development of the enteric nervous system. Prog. Brain Res. 2004, 146, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-B.; Zuo, X.-L.; Zhao, Q.-J.; Chen, F.-X.; Yang, J.; Dong, Y.-Y.; Wang, P.; Li, Y.-Q. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut 2011, 61, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, J.; Bowen, E.J.; Russo, A.F.; Durham, P.L. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur. J. Neurosci. 2006, 23, 2057–2066. [Google Scholar] [CrossRef] [Green Version]
- Banks, W.A. The blood-brain barrier: Connecting the gut and the brain. Regul. Pept. 2008, 149, 11–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanza, M.; Filippone, A.; Ardizzone, A.; Casili, G.; Paterniti, I.; Esposito, E.; Campolo, M. SCFA Treatment Alleviates Pathological Signs of Migraine and Related Intestinal Alterations in a Mouse Model of NTG-Induced Migraine. Cells 2021, 10, 2756. https://doi.org/10.3390/cells10102756
Lanza M, Filippone A, Ardizzone A, Casili G, Paterniti I, Esposito E, Campolo M. SCFA Treatment Alleviates Pathological Signs of Migraine and Related Intestinal Alterations in a Mouse Model of NTG-Induced Migraine. Cells. 2021; 10(10):2756. https://doi.org/10.3390/cells10102756
Chicago/Turabian StyleLanza, Marika, Alessia Filippone, Alessio Ardizzone, Giovanna Casili, Irene Paterniti, Emanuela Esposito, and Michela Campolo. 2021. "SCFA Treatment Alleviates Pathological Signs of Migraine and Related Intestinal Alterations in a Mouse Model of NTG-Induced Migraine" Cells 10, no. 10: 2756. https://doi.org/10.3390/cells10102756