Ferulic Acid in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Methodological Quality of Studies
2.5. Statistical Analysis
3. Results
3.1. Literature Screening
3.2. FA Treatment Alleviates AD-Related Behaviors
3.3. FA Treatment Improves Neuropathological Features in AD Animal Models
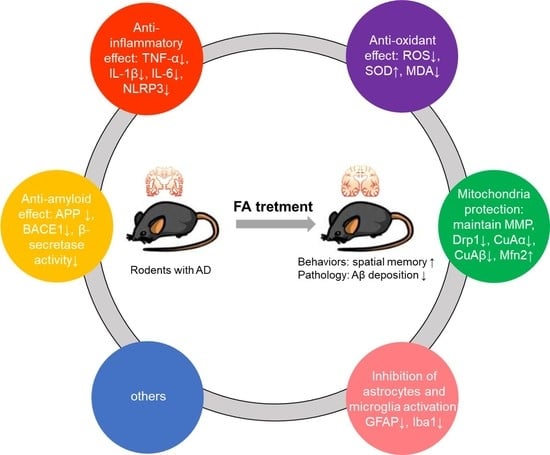
3.4. Mechanism of FA in Anti-AD
4. Discussion
4.1. Article Characteristics
4.2. Anti-AD Potential of FA
4.3. Possible Mechanisms of FA in the Treatment of AD
4.3.1. Anti-Amyloid Effect
4.3.2. Anti-Inflammatory Effect
4.3.3. Antioxidant Effect
4.3.4. Mitochondria Protection
4.3.5. Inhibition of Astrocytes and Microglia Activation
4.3.6. Others
4.4. Development Perspective
4.4.1. Mechanism Exploration
4.4.2. Structural Modification
4.4.3. Extrapolation to Humans
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, M.J.; Wimo, A.; Guerchet, M.M.; Ali, G.C.; Wu, Y.T.; Prina, M. World Alzheimer Report 2015. The Global Impact of Dementia. An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015; pp. 10–11. [Google Scholar]
- Shah, H.; Albanese, E.; Duggan, C.; Rudan, I.; Langa, K.M.; Carrillo, M.C.; Chan, K.Y.; Joanette, Y.; Prince, M.; Rossor, M. Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol. 2016, 15, 1285–1294. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Ferulic Acid: An Antioxidant Found Naturally in Plant Cell Walls and Feruloyl Esterases Involved in its Release and Their Applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.G.; Harris, P.J. Ferulic acid is esterified to glucuronoarabinoxylans in pineapple cell walls. Phytochemistry 2001, 56, 513–519. [Google Scholar] [CrossRef]
- Yun, L.; Zhao, H.P.; Jing, Z.; Jie, W.; Liao, Z.G. Effect of ferulic acid on learning and memory impairments of vascular dementia rats and its mechanism of action. Acta Pharm. Sin. 2012, 47, 256–260. [Google Scholar] [CrossRef]
- Wu, J.L.; Shen, M.M.; Yang, S.X.; Wang, X.; Ma, Z.C. Inhibitory effect of ferulic acid on neuroinflammation in LPS-activated microglia. Chin. Pharmacol. Bull. 2015, 31, 97–102. [Google Scholar]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Macleod, M.R.; O’Collins, T.; Howells, D.W.; Donnan, G.A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 2004, 35, 1203–1208. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Merel, R.-H.; Langendam, M.W. Systematic reviews of animal experiments. BMC Med. Res. Methodol. 2014, 14, 586. [Google Scholar] [CrossRef]
- Yan, J.J.; Cho, J.Y.; Kim, H.S.; Kim, K.L.; Jung, J.S.; Huh, S.O.; Suh, H.W.; Kim, Y.H.; Song, D.K. Protection against β-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br. J. Pharmacol. 2001, 133, 89–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Cho, J.Y.; Kim, D.H.; Yan, J.J.; Lee, H.K.; Suh, H.W.; Song, D.K. Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of β-amyloid peptide (1–42) in mice. Biol. Pharm. Bull. 2004, 27, 120–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.-Y.; Kim, H.-S.; Kim, D.-H.; Yan, J.-J.; Suh, H.-W.; Song, D.-K. Inhibitory effects of long-term administration of ferulic acid on astrocyte activation induced by intracerebroventricular injection of beta-amyloid peptide (1-42) in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, T.; Kise, M.; Morikawa, K. Ferulic acid attenuated cognitive deficits and increase in carbonyl proteins induced by buthionine-sulfoximine in mice. Neurosci. Lett. 2008, 430, 115–118. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-beta aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beibei, J.; Qin, C.; Qinglin, C. Effect of Ferulic Acid on Learing-memory and Expression of GFAP in the Hippocampus Tissue of Alzheimer’s Disease-like Modle Mice. Acta Laser Biol. Sin. 2011, 20, 484–489. [Google Scholar] [CrossRef]
- Yan, J.-J.; Jung, J.-S.; Kim, T.-K.; Hasan, A.; Hong, C.-W.; Nam, J.-S.; Song, D.-K. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol. Pharm. Bull. 2013, 36, 140–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.-V.; Tan, J.; Town, T. Ferulic acid is a nutraceutical beta-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS ONE 2013, 8, e55774. [Google Scholar] [CrossRef] [Green Version]
- Tsai, F.-S.; Wu, L.-Y.; Yang, S.-E.; Cheng, H.-Y.; Tsai, C.-C.; Wu, C.-R.; Lin, L.-W. Ferulic acid reverses the cognitive dysfunction caused by amyloid beta peptide 1-40 through anti-oxidant activity and cholinergic activation in rats. Am. J. Chin. Med. 2015, 43, 319–335. [Google Scholar] [CrossRef]
- Huang, H. The Anti-Alzheimer Disease Mechanism of Ferulic Acid as the Main Target of Phosphodiesterase. Ph.D. Thesis, Beijing University of Technology, Beijing, China, 2016; pp. 1–131. [Google Scholar]
- Kikugawa, M.; Tsutsuki, H.; Ida, T.; Nakajima, H.; Ihara, H.; Sakamoto, T. Water-soluble ferulic acid derivatives improve amyloid-beta-induced neuronal cell death and dysmnesia through inhibition of amyloid-beta aggregation. Biosci. Biotechnol. Biochem. 2016, 80, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combination therapy with octyl gallate and ferulic acid improves cognition and neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 2017, 292, 11310–11325. [Google Scholar] [CrossRef] [Green Version]
- Yue, W.; Xu, W.; Song, Y.U.; Chun, W. Effects of Ferulic Acid on Oxidative Stress and Apoptosis Related Proteins in Alzheimer’s Disease Transgenic Mice. Nat. Prod. Res. Dev. 2017, 29, 762–766. [Google Scholar] [CrossRef]
- Rui, M.; Yi-qing, C.; Qin, C. Effects of ferulic acid on glial activation and inflammatory cytokines expression in the cerebral cortex of Alzheimer’s disease like model mice. Chin. Hosp. Pharm. J. 2018, 38, 50–53. [Google Scholar] [CrossRef]
- Zafeer, M.F.; Firdaus, F.; Anis, E.; Mobarak Hossain, M. Prolong treatment with Trans-ferulic acid mitigates bioenergetics loss and restores mitochondrial dynamics in streptozotocin-induced sporadic dementia of Alzheimer’s type. Neurotoxicology 2019, 73, 246–257. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combined treatment with the phenolics (-)-epigallocatechin-3-gallate and ferulic acid improves cognition and reduces Alzheimer-like pathology in mice. J. Biol. Chem. 2019, 294, 2714–2731. [Google Scholar] [CrossRef] [Green Version]
- Qian, W.; Wei-wei, Q.; Jie-wen, Z. Effect of Ferulic Acid on Learning and Memory Impairment by the Repairing of Mitochondrial Fission-Fusion Imbalance in AD Mice. Chin. Pharm. J. 2019, 54, 703–710. [Google Scholar]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Sarter, M.; Bodewitz, G.; Stephens, D.N. Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology 1988, 94, 491–495. [Google Scholar] [CrossRef]
- Westerman, M.A.; Cooperblacketer, D.; Mariash, A.; Kotilinek, L.; Kawarabayashi, T.; Younkin, L.H.; Carlson, G.A.; Younkin, S.G.; Ashe, K.H. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J. Neurosci. 2002, 22, 1858–1867. [Google Scholar] [CrossRef] [Green Version]
- Cleary, J.P.; Walsh, D.M.; Hofmeister, J.J.; Shankar, G.M.; Ashe, K.H. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat. Neurosci. 2005, 8, 79–84. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Selkoe, D.J. Amyloid β-Protein Dimers Isolated Directly from Alzheimer Brains Impair Synaptic Plasticity and Memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.Y.; Cui, X.Y. Behavioral Study on Learning and Memory Ability of BALB/c and ICR Mice. Acta Lab. Anim. Sci. Sin. 2007, 5, 372–375. [Google Scholar]
- Liu, G.; Zeng-Yao, H.U.; Yang, S.; Zhou, W.X.; Zhang, Y.X. Comparison of Alzheimer’s disease animal model in BALB/c and Kunming mice by intracerebroventricular injection of β-amyloid. Bull. Acad. Mil. Med. Sci. 2009, 33, 554–557. [Google Scholar]
- Ono, K.; Hirohata, M.; Yamada, M. Ferulic acid destabilizes preformed beta-amyloid fibrils in vitro. Biochem. Biophys. Res. Commun. 2005, 336, 444–449. [Google Scholar] [CrossRef]
- Jagota, S.; Rajadas, J. Effect of phenolic compounds against Abeta aggregation and Abeta-induced toxicity in transgenic C. elegans. Neurochem. Res. 2012, 37, 40–48. [Google Scholar] [CrossRef]
- Yan, R.Q.; Bienkowski, M.J.; Shuck, M.E.; Miao, H.Y.; Gurney, M.E. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature 1999, 402, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Goldsbury, C.; Whiteman, I.T.; Jeong, E.V.; Lim, Y.A. Oxidative stress increases levels of endogenous amyloid-β peptides secreted from primary chick brain neurons. Aging Cell 2008, 7, 771–775. [Google Scholar] [CrossRef]
- Pappolla, M.A.; Chyan, Y.J.; Omar, R.A.; Hsiao, K.; Bozner, P. Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer’s disease: A chronic oxidative paradigm for testing antioxidant therapies in vivo. Am. J. Pathol. 1998, 152, 871–877. [Google Scholar] [PubMed]
- William, R. Markesbery Oxidative Stress Hypothesis in Alzheimer’s Disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Amyloid β-Peptide(1-42) Contributes to the Oxidative Stress and Neurodegeneration Found in Alzheimer Disease Brain. Brain Pathol. 2006, 14, 426–432. [Google Scholar] [CrossRef]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Lin, X.; Wang, D.; Zhang, Z.; Guo, Y.; Ren, X.; Xu, B.; Yuan, J.; Liu, J.; Spencer, P.S.; et al. Mitochondrial Molecular Abnormalities Revealed by Proteomic Analysis of Hippocampal Organelles of Mice Triple Transgenic for Alzheimer Disease. Front. Mol. Neurosci. 2018, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Tammineni, P. Mitochondrial Aspects of Synaptic Dysfunction in Alzheimer’s Disease. J. Alzheimers Dis. 2016, 57, 1087–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.L.; Shen, Y.; Wang, X.; Wei, L.F.; Wang, P.; Yang, H.; Wang, C.F.; Xie, Z.H.; Bi, J.Z. Mitochondrial dynamics changes with age in an APPsw/PS1dE9 mouse model of Alzheimer’s disease. Neuroreport 2017, 28, 222–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, P.H.; Reddy, T.P.; Manczak, M.; Calkins, M.J.; Shirendeb, U.; Mao, P. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res. Rev. 2011, 67, 103–118. [Google Scholar] [CrossRef] [Green Version]
- Nitta, A.; Fukuta, T.; Hasegawa, T.; Nabeshima, T. Continuous Infusion of BETA-Amyloid Protein into the Rat Cerebral Ventricle Induces Learning Impairment and Neuronal and Morphological Degeneration. Jpn. J. Pharmacol. 1997, 73, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Egashira, Y.; Sanada, H. Ferulic Acid Is Quickly Absorbed from Rat Stomach as the Free Form and Then Conjugated Mainly in Liver. J. Nutr. 2004, 134, 3083–3088. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010, 13, 1763. [Google Scholar] [CrossRef] [PubMed]











| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Rodent with a clear genetic origin | Cell model or non-rodent |
| Included a ferulic acid group and a control group administered by any route, and each group was independent of the other group. | Groups without ferulic acid treatment or no control group |
| AD model or contains AD model | Not AD model |
| Study that assessed AD-related results, such as behavioral changes and protein changes. | Study that did not assess AD-related results |
| Full access to published study | Unable to access full text, review, case report, editorial, abstract, letter, and/or comments |
| Study | Animal Models and Species | Quantity Sex Age | Administration | Outcome | ||
|---|---|---|---|---|---|---|
| Behavioral Change | Neuropathological Change | Biochemical Change | ||||
| Ji-Jing Yan 2001 [11] | i.c.v. injection of Aβ1-42 ICR mice | 10 M 18~26 g | 0.002%, 0.004%, and 0.006% (w/v) Free drinking 1, 2, 3, 4 w | Improved memory (passive avoidance task; Y-maze tests; MWM) | Hippocampus GFAP and IL-1β immunoreactivities↑ (Immunocytochemistry) | Cortex Acetylcholine level↓ (colorimetry); |
| Hee-Sung KIM 2004 [12] | i.c.v. injection of Aβ1-42 ICR mice | 6 M 18~26 g | 0.006% (w/v) Free drinking 4 w | N/A? | Reduced microglial activation (Immunocytochemistry: OX-42 immunoreactivity↓) | Inhibition IFN-γ Immunoreactivity (Immunocytochemistry); |
| Jae-Young Cho 2005 [13] | i.c.v. injection of Aβ1-42 ICR mice | 6 M 18~26 g | 0.006% (w/v) Free drinking 4 w | N/A? | Reduced astrocytes activation | Alleviated oxidative stress in the hippocampus (eNOS and 3- NT immunoreactivity↓) |
| Takayoshi Mamiya 2008 [14] | i.c.v. injection of BSO ICR mice | 10/15 M 25 w | 0.5, 1, or 5 mg/kg sc 6 d | Improve recognition memory (the novel object recognition test); improve short-term memory (Y-maze); | extent of protein oxidation↓; carbonyl protein levels↓ in forebrains; | N/A? |
| Tsuyoshi Hamaguchi 2009 [15] | Mice double mutation K670N-M671L Tg2576 mice | 10 F 5 mon | 0.5% in food 10 mon | N/A? | Aβ deposits↓ (IHC) | N/A? |
| JIN Beibei 2011 [16] | Injected KA into hippocampus CA1 region KM mice | 10 M&F 20~30 g | 20, 40 and 80 mg/kg ig 30 d | Improved learning and cognitive skills (MWM) | Reduced expressions of GFAP in hippocampal CA1 region (Immunohistochemistry) | N/A? |
| Ji-Jing Yan 2013 [17] | APP/PS1 mice | 5 F 6 mon | 5.3 and 16 mg/kg/d Free drinking 6 mon | Improved memory (novel-object recognition test, Y-maze task) | Aβ1-42 and Aβ1-40 levels↓ (immunoassay kits) | Il-1β↓ (immunoassay kits) |
| Takashi Mori 2013 [18] | PSAPP C57BL/6J mice | 12 M&F 6 mon | 30 mg/kg ig 6 mon | Remediation of behavioral impairment (field activity testing; object recognition test; Y-maze test; MWM) | Cerebral Aβ deposits↓ (4G8 immunohistochemistry, ELISA) | Reduced neuroinflammation and Oxidative Stress: Iba1↑ (Immunohistochemistry); TNF-a, IL-1β, Sod1, catalase, and Gpx1 mRNA↓ (QRT-PCR)↓; reduced microglial and astroglial activation:GFAP↓ (Immunohistochemistry) |
| Fan-Shiu Tsai 2015 [19] | i.c.v. injection of Aβ1-42 SD rats | 10~12 M 250~300 g | 50 and 100 mg/kg ig 2 w | Attenuated impairment of cognitive function (Inhibitory Avoidance Test); improve memory (MWM); | N/A? | Cortical and hippocampal GSH↑, SOD↑, Cu, Zn-SOD↓ activity (spectrophotometrically); brain AChE Activity↓ (Ellman method) |
| Huang Hao 2016 [20] | LPS-induced SD rats | 12 F 280~320 g | 25, 50, 100 mg/kg ig 34 d | Improved learning and cognitive skills (MWM) | Protective effect on brain histopathology (HE staining, β-tubulin), PDE4B | Anti-oxidize effect (SOD↑); suppressed mRNA elevation of PDE4B, NLRP3, IL-1β and caspase-1(Q-PCR); PDE4B↓ (Immunohistochemistry, WB); NLRP3↓, CREB↑ and pCREB↑ (WB) |
| Masaki Kikugawa 2016 [21] | i.c.v. injection of Aβ25~35 C57BL/6 J mice | 6 M 16–19 g | 0.1 μmol/g/day po 42 d | Improved contextual freezing response impairment (fear conditioning test) | Protective effects on neurons survival (Nissl stain) | N/A? |
| Takashi Mori 2017 [22] | APP/PS1 C57BL/6J mice | 8 M&F 12 mon | 30 mg/kg ig 3 mon | Improved memory (assess novel object recognition memory, the novel object recognition test and retention test phases; Y-maze test, RAWM) | Cerebral parenchymal A β deposits↓and size↓ (IHC), A β 1-40, A β 1-42↓ (ELISA); vascular A β deposits↓ (IHC); attenuated astrocytosis and microgliosis (IHC of GFAP and Iba1); Attenuated Synaptotoxicity: synaptophysin immunoreactivity↑ (IHC) | Promoted nonamyloidogenic and inhibited amyloidogenic APP processing: sAPP-α/holo-APP↓ (WB), β-oligomers↓ (ELISA); activated ADAM10 and inhibits BACE1(WB); attenuated neuroinflammation and oxidative stress: TNF-α↓, IL-1β↓, SOD1↓, GPx1↓ (Q-PCR); attenuated Synaptotoxicity: synaptophysin immunoreactivity↑ (IHC) |
| Wang Yue 2017 [23] | APP/PS1 C57BL/6 mice | 10 15~20 g | 20, 40, 100 mg/kg ig 7 d | N/A? | N/A? | Reduced apoptosis (WB: Bcl-2↑, Bax↓, p-JNK↓, p-C-Jun↓, Caspase3↓), Reduces oxidative stress in the brain (MDA↓, SOD↑) |
| MING Rui 2018 [24] | Injected KA into hippocampus CA1 region KM mice | M&F 26 ± 4 g | 20, 40, and 80 mg/kg 30 d | N/A? | Reduced number of positive GFAP cells in cerebral cortical glial cells (Immunofluorescence) | Reduced inflammatory cytokines (ELISA: IL-1β↓, IL-6↓, TNF-α↓) |
| Mohd Faraz Zafeer 2019 [25] | ICV-STZ Wistar rats | 6 M 350 ± 25 g | 100 mg/kg po 21 d | Attenuated spatial memory and learning loss (MWM) | Protective effect on brain histopathology (HE staining of coronal sections) | Mitigation of AD-related oxidative stress (DCFDA: ROS↓); mito-protective efficacy (flow cytometric: Δψm; Calcein-AM/CoCl2 assay: mPTP; WB: Drp-1↑, Mfn2↓, PGC1-α↑, BAX↓, Cytochrome-C↓, LPO↓); DNA fragmentation↓ (comet assay) |
| Takashi Mori 2019 [26] | APP/PS1 mice | 8 M&F 12 mon | 30 mg/kg ig 3 mon | Improved memory (Y-maze, RAWM; novel object recognition test; alternation Y-maze task) | Cerebral Aβ deposits↓ (4G8 immunostain); Aβ1-40 and Aβ1-42 levels↓ (ELISA) | Promoted nonamyloidogenic and inhibited amyloidogenic APP cleavage (WB); ADAM10 ↓, BACE1 ↓ (WB); mitigated astrocytosis and microgliosis (IHC of GFAP and Iba1); dampened neuroinflammation and oxidative stress: TNF-α↓, IL-1β↓ (Q-PCR), SOD1↓, GPx1↓ (Q-PCR and WB); attenuated Synaptotoxicity: synaptophysin immunoreactivity↑ (IHC) |
| WANG Qian 2019 [27] | Injecting Aβ1-42 into the lateral ventricle KM mice | 10 M 18~22 g | 0.1 and 0.4 g/kg ig | Improved spatial positioning memory (MWM). No effect on the excitability of the central nervous system (spontaneous activity experiment) | Improved morphological changes (HE Staining); Tau; pS396 protein phosphorylated, total Tau protein↓ and S396↓; reduced Aβ generation | Improved abnormal mitochondrial division (RT-PCR: Drp1↓, CnAα↓, CnAβ↓mRNA); Bace1↓ |
| Study | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | Quality Score | Quality Score (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ji-Jing Yan 2001 | √ | × | √ | × | × | × | √ | √ | √ | √ | 6 | 60 |
| Hee-Sung KIM 2004 | √ | × | × | × | × | × | √ | √ | √ | √ | 5 | 50 |
| Jae-Young Cho 2005 | √ | × | × | × | × | × | √ | √ | √ | √ | 5 | 50 |
| Takayoshi Mamiya 2008 | √ | × | √ | × | × | × | √ | √ | √ | √ | 6 | 60 |
| Tsuyoshi Hamaguchi 2009 | √ | √ | × | × | × | × | √ | √ | √ | √ | 6 | 60 |
| JIN Beibei 2011 | √ | × | √ | × | × | × | √ | √ | √ | √ | 6 | 60 |
| Ji-Jing Yan 2013 | √ | × | √ | × | × | × | √ | √ | √ | √ | 6 | 60 |
| Takashi Mori 2013 | √ | × | × | × | × | × | √ | √ | √ | √ | 5 | 50 |
| Fan-Shiu Tsai 2015 | √ | √ | √ | √ | × | × | √ | √ | √ | √ | 8 | 80 |
| Haung Hao 2016 | √ | √ | √ | × | × | × | √ | √ | √ | √ | 7 | 70 |
| Masaki Kikugawa 2016 | √ | × | × | × | × | × | √ | √ | √ | √ | 5 | 50 |
| Takashi Mori 2017 | √ | × | × | × | × | × | √ | √ | √ | √ | 5 | 50 |
| Wang Yue 2017 | √ | √ | √ | × | × | × | √ | √ | √ | √ | 7 | 70 |
| MING Rui 2018 | √ | × | √ | × | × | × | √ | √ | √ | √ | 6 | 60 |
| Mohd Faraz Zafeer 2019 | √ | × | × | × | × | × | √ | √ | √ | √ | 5 | 50 |
| Takashi Mori 2019 | √ | √ | × | √ | × | × | √ | √ | √ | √ | 7 | 70 |
| WANG Qian 2019 | √ | √ | √ | × | × | × | √ | √ | √ | √ | 7 | 70 |
| Pharmacological Effects | Mechanism | Studys |
|---|---|---|
| Anti-amyloid effect | Inhibition of Aβ deposition | [17] |
| Inhibition of the formation and extension of Aβ | [35,36] | |
| Inhibition of β-secretase | [18,22,26,27] | |
| Reduce APP and Tau expression | [27] | |
| Anti-inflammatory effect | Reduce TNF-a, IL-6 and IL- 1β expression | [17,18,20,22,24,26] |
| Block the activity of NLRP3 inflammasome | [20] | |
| Antioxidant effect | Inhibition ROS and MDA production, increase SOD expression | [14,18,19,20,22,23,25,42] |
| Mitochondria protection | reverse the abnormally increased expression of Drp1 | [25,27] |
| Inhibition of astrocytes and microglia activation | Reduce GFAP positive astrocytes | [11,16,18,22,24,25,26] |
| Reduce eNOS, 3-NT in astrocytes | [13] | |
| Reduce Iba1 positive microglia | [12,18,22,26] | |
| Others | Inhibition AChE activity | [19] |
| Reducing the phosphorylation of apoptosis-related proteins | [23,25] | |
| Regulate PDE4/cAMP/CREB signaling pathway | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, E.-J.; Wu, M.-Y.; Lu, J.-H. Ferulic Acid in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Cells 2021, 10, 2653. https://doi.org/10.3390/cells10102653
Wang E-J, Wu M-Y, Lu J-H. Ferulic Acid in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Cells. 2021; 10(10):2653. https://doi.org/10.3390/cells10102653
Chicago/Turabian StyleWang, Er-Jin, Ming-Yue Wu, and Jia-Hong Lu. 2021. "Ferulic Acid in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies" Cells 10, no. 10: 2653. https://doi.org/10.3390/cells10102653