Comparison of the Virulence of Space Mutants of Aspergillus oryzae XJ-1 against Adult Locusta migratoria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening and Identification of A. oryzae XJ-1 Mutants
2.2. Comparison of the Virulence of A. oryzae Mutants and XJ-1 against Adult L. migratoria
2.3. Comparison of the Optimal Growth Temperature of TQ549 and A. oryzae XJ-1
2.4. Statistical Analysis
3. Results
3.1. Screening and Identification of XJ-1 Mutants
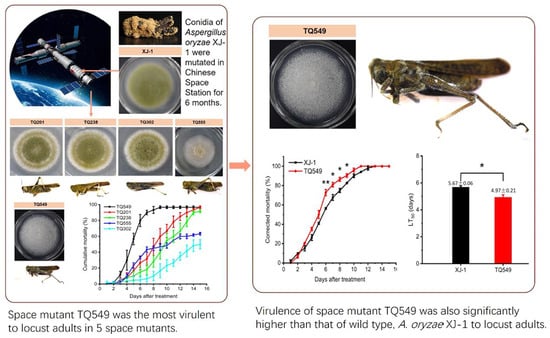
3.2. Virulence of Mutants against L. migratoria Adults in the Laboratory
3.3. Comparison of the Growth Rates of TQ549 and A. oryzae XJ-1 at Different Temperatures
4. Discussion
5. Conclusions
- Five mutants were screened from A. oryzae XJ-1 generated by space mutagenesis, and the morphological characteristics of their colonies on PDA plates differed from that of A. oryzae XJ-1. Analysis of their ITS sequences indicated that all these mutants belonged to A. oryzae.
- Experiments on the mortality rates of these mutants and A. oryzae XJ-1 against L. migratoria adults showed that only one mutant, TQ549, exhibited higher virulence than the original strain, A. oryzae XJ-1, and the virulences of the other four mutants were all lower than that of A. oryzae XJ-1.
- Our results indicated that space mutant TQ549, which had white conidia, high virulence against adult locusts, and tolerance of a wide temperature range, had the potential to be used as a biological agent for the control of locusts.
- These mutants with different virulence against adult locusts will provide us with good materials to research the pathogenic molecular mechanism of A. oryzae XJ-1 by comparison of genomes and transcriptomes of A. oryzae XJ-1 and the mutants.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D. Locust and grasshopper management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Lecoq, M.; Zhang, L. Encyclopedia of Pest Orthoptera of the World; China Agricultural University Press: Beijing, China, 2019. [Google Scholar]
- Bennett, L.V. Development of a desert locust plague. Nature 1975, 256, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Lomer, C.J.; Bateman, R.P.; Johnson, D.L.; Langewald, J.; Thomas, M. Biological control of locusts and grasshoppers. Annu. Rev. Entomol. 2001, 46, 667–702. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- Brooks, W.M. Entomogenous protozoa. In CRC Handbook of Natural Pesticides. Microbial Insecticides, Part A: Entomogenous Protozoa and Fungi; Ignoffo, C.M., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Volume 5, pp. 1–49. [Google Scholar]
- Henry, J.E.; Oma, E.A. Pest control by Nosema locustae, a pathogen of grasshoppers and crickets. In Microbial Control of Pests and Plant Diseases (1970–1980); Burges, H.D., Ed.; Academic Press: New York, NY, USA, 1981; pp. 573–586. [Google Scholar]
- Henry, J.E. Epizootiology of infections by Nosema locustae Canning (Microsporidia: Nosematidae) in grasshoppers. Acrida 1972, 1, 111. [Google Scholar]
- Henry, J.E. Experimental application of Nosema locustae for control of grasshoppers. J. Invertebr. Pathol. 1971, 18, 389. [Google Scholar] [CrossRef]
- Henry, J.E.; Tiahrt, K.; Omaha, E.A. Importance of timing, spore concentrations, and levels of spore carrier in application of Nosema locustae (Microsporida: Nosematidae) for control of grasshoppers. J. Invertebr. Pathol. 1973, 21, 263. [Google Scholar] [CrossRef]
- Lomer, C.J.; Prior, C.; Kooyman, C. Development of Metarhizium spp. for the control of grasshoppers and locusts. Mem. Entomol. Soc. Can. 1997, 129, 265–286. [Google Scholar] [CrossRef]
- Streett, D.A.; Woods, S.A.; Erlandson, M.A. Entomopoxviruses of grasshoppers and locusts: Biology and biological control potential. Mem. Entomol. Soc. Can. 1997, 129, 115–130. [Google Scholar] [CrossRef]
- Bateman, R.; Carey, M.; Batt, D.; Prior, C.; Abraham, Y.; Moore, D.; Jenkins, N.; Fenlon, J. Screening for virulent isolates of entomopathogenic fungi against the desert locust, Schistocerca gregaria Forskal. Biocontrol Sci. Technol. 1996, 6, 549–560. [Google Scholar] [CrossRef]
- Jaronski, S.T.; Goettel, M.S. Development of Beauveria bassiana for control of grasshoppers and locusts. Mem. Entomol. Soc. Can. 1997, 129, 225–237. [Google Scholar] [CrossRef]
- Inglis, G.; Johnson, D.L.; Goettel, M.S. Field and laboratory evaluation of two conidial batches of Beauveria bassiana (balsamo) vuillemin against grasshoppers. Can. Entomol. 1997, 129, 171–186. [Google Scholar] [CrossRef]
- Zhong, Y.Y.; Lu, X.; Xing, L.; Ho, S.W.A.; Kwan, H.S. Genomic and transcriptomic comparison of Aspergillus oryzae strains: A case study in soy sauce koji fermentation. J. Ind. Microbiol. Biot. 2018, 45, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.V.; Joshi, K.R.; Harjai, S.C.; Ramdeo, I.N. Aspergillosis in desert locust (Schistocerka gregaria Forsk). Mycopathologia 1975, 57, 135–138. [Google Scholar] [CrossRef]
- Zhang, P.; You, Y.; Song, Y.; Wang, Y.; Zhang, L. First record of Aspergillus oryzae (Eurotiales: Trichocomaceae) as an entomopathogenic fungus of the locust, Locusta migratoria (Orthoptera: Acrididae). Biocontrol Sci. Technol. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- You, Y.; An, Z.; Zhang, X.; Liu, H.; Yang, W.; Yang, M.; Wang, T.; Xie, X.; Zhang, L. Virulence of the fungal pathogen, Aspergillus oryzae XJ-1 to adult locusts (Orthoptera: Acrididae) in both laboratory and field trials. Pest Manag. Sci. 2023, 79, 3767–3772. [Google Scholar] [CrossRef]
- Suryadi, H.; Irianti, M.I.; Septiarini, T.H. Methods of random mutagenesis of Aspergillus strain for increasing kojic acid production. Curr. Pharm. BiotechnoI. 2022, 23, 486–494. [Google Scholar] [CrossRef]
- Milojevic, T.; Weckwerth, W. Molecular mechanisms of microbial survivability in outer space: A systems biology approach. Front. Microbiol. 2020, 11, 923. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Liu, J.; Fang, X.; Xu, C.; Guo, Y.; Chang, D.; Su, L. Space mutagenesis of genetically engineered bacteria expressing recombinant human interferon α1b and screening of higher yielding strains. World J. Microbiol. Biotechnol. 2014, 30, 943–949. [Google Scholar] [CrossRef]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space Microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121–156. [Google Scholar] [CrossRef]
- Acres, J.M.; Youngapelian, M.J.; Nadeau, J. The influence of spaceflight and simulated microgravity on bacterial motility and chemotaxis. NPJ Microgravity 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Blachowicz, A.; Chiang, A.J.; Romsdahl, J.; Kalkum, M.; Wang, C.C.C.; Venkateswaran, K. Proteomic characterization of Aspergillus fumigatus isolated from air and surfaces of the International Space Station. Fungal Genet. Biol. 2019, 124, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Cary, J.W.; Harris-Coward, P.Y.; Ehrlich, K.C.; Di Mavungu, J.D.; Malysheva, S.V.; De Saeger, S.; Dowd, P.F.; Shantappa, S.; Martens, S.L.; Calvo, A.M. Functional characterization of a veA-dependent polyketide synthase gene in Aspergillus flavus necessary for the synthesis of asparasone, a sclerotium-specific pigment. Fungal Genet. Biol. 2014, 64, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.S. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 2000, 13, 708–717. [Google Scholar] [CrossRef]
- Akoumianaki, T.; Kyrmizi, I.; Valsecchi, I.; Gresnigt, M.S.; Samonis, G.; Drakos, E.; Boumpas, D.; Muszkieta, L.; Prevost, M.C.; Kontoyiannis, D.P.; et al. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity. Cell Host Microbe 2016, 19, 79–90. [Google Scholar] [CrossRef]
- Kyrmizi, I.; Ferreira, H.; Carvalho, A.; Figueroa, J.A.L.; Zarmpas, P.; Cunha, C.; Akoumianaki, T.; Stylianou, K.; Deepe, G.S., Jr.; Samonis, G.; et al. Calcium sequestration by fungal melanin inhibits calcium-calmodulin signalling to prevent LC3-associated phagocytosis. Nat. Microbiol. 2018, 3, 791–803. [Google Scholar] [CrossRef]
- Anonymous. Funding gap for locust crisis. Nat. Food 2020, 1, 143. [Google Scholar]
- Zhang, L.; Lecoq, M. Nosema locustae (Protozoa, Microsporidia), a biological agent for locust and grasshopper control. Agronomy 2021, 11, 711. [Google Scholar] [CrossRef]





| Strain | Length (bp) | Result |
|---|---|---|
| TQ201 | 595 | 99.66% identity with A. oryzae XJ-1 |
| TQ238 | 595 | 99.66% identity with A. oryzae XJ-1 |
| TQ302 | 595 | 99.50% identity with A. oryzae XJ-1 |
| TQ549 | 595 | 99.66% identity with A. oryzae XJ-1 |
| TQ555 | 595 | 99.66% identity with A. oryzae XJ-1 |
| Days | 24 °C | 33 °C | 38 °C | |||
|---|---|---|---|---|---|---|
| A. oryzae XJ-1 | TQ549 | A. oryzae XJ-1 | TQ549 | A. oryzae XJ-1 | TQ549 | |
| 1 | 6.20 ± 0.37 a | 7.25 ± 0.37 a | 13.85 ± 0.31 a | 14.00 ± 0.22 a | 8.44 ± 0.26 a | 8.88 ± 0.13 a |
| 2 | 20.60 ± 0.40 a | 21.38 ± 0.26 a | 33.25 ± 0.41 a | 33.00 ± 0.26 a | 25.38 ± 0.18 a | 23.88 ± 0.31 b |
| 3 | 34.80 ± 0.20 a | 35.25 ± 0.16 a | 53.75 ± 0.56 a | 51.30 ± 0.20 b | 35.75 ± 0.16 a | 33.63 ± 0.13 b |
| 4 | 47.80 ± 0.20 a | 47.13 ± 0.48 a | 70.81 ± 0.53 a | 67.20 ± 0.20 b | 43.50 ± 0.27 a | 40.50 ± 0.29 b |
| 5 | 60.00 ± 0.55 a | 59.38 ± 0.84 a | 82.50 ± 0.50 a | 80.80 ± 0.49 b | 51.25 ± 0.46 a | 48.50 ± 0.87 a |
| 6 | 70.60 ± 0.40 a | 69.25 ± 1.17 a | 82.50 ± 0.50 a | 83.40 ± 0.40 a | 59.00 ± 0.34 a | 56.87 ± 1.05 a |
| 7 | 74.60 ± 0.51 a | 74.50 ± 0.73 a | 84.00 a | 83.60 ± 0.10 a | 67.13 ± 0.67 a | 66.00 ± 1.41 a |
| 8 | 75.20 ± 0.37 a | 78.50 ± 1.51 a | 84.00 a | 84.00 a | 77.75 ± 0.49 a | 72.88 ± 1.09 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Liu, H.; Xu, X.; Guo, J.; Hu, S.; You, Y.; Zhang, L. Comparison of the Virulence of Space Mutants of Aspergillus oryzae XJ-1 against Adult Locusta migratoria. Agronomy 2024, 14, 116. https://doi.org/10.3390/agronomy14010116
Fu X, Liu H, Xu X, Guo J, Hu S, You Y, Zhang L. Comparison of the Virulence of Space Mutants of Aspergillus oryzae XJ-1 against Adult Locusta migratoria. Agronomy. 2024; 14(1):116. https://doi.org/10.3390/agronomy14010116
Chicago/Turabian StyleFu, Xin, Hui Liu, Xiao Xu, Jin Guo, Shaojing Hu, Yinwei You, and Long Zhang. 2024. "Comparison of the Virulence of Space Mutants of Aspergillus oryzae XJ-1 against Adult Locusta migratoria" Agronomy 14, no. 1: 116. https://doi.org/10.3390/agronomy14010116