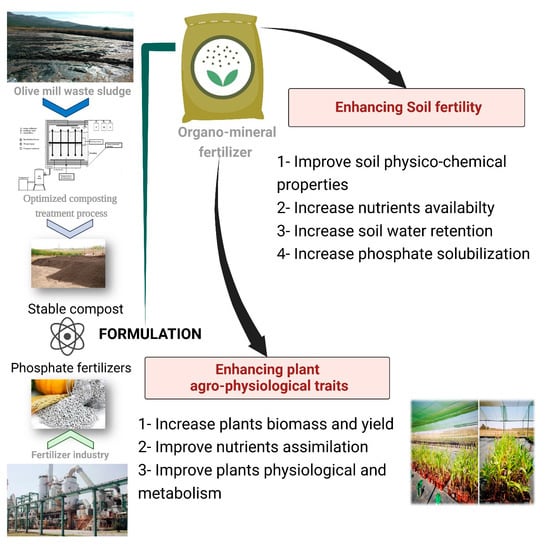
Organo-Mineral Fertilization Based on Olive Waste Sludge Compost and Various Phosphate Sources Improves Phosphorus Agronomic Efficiency, Zea mays Agro-Physiological Traits, and Water Availability
Abstract
:1. Introduction
2. Material and Methods
2.1. Sampling of Raw Materials and Compost Preparation
2.2. Plant Material and Experimental Design
2.3. Soil Chemical Analysis
2.4. Plant Biomass and Agro-Physiological Traits
2.5. Yields, Harvest Index (HI), and Water Use Efficiency (WUE)
2.6. Plant Nutrient Analysis
2.7. Phosphorus Use Efficiency-Related Parameters
2.8. Statistical Analysis
3. Results
3.1. Effect of OMF Application on Initial Soil Physicochemical Properties
3.2. Effect of OMF Application on Soil Physicochemical Properties Post-Harvesting
3.3. PUE and PSR
3.4. Effect of OMF Formulation on Plant’s Agro-Physiological Parameters and Nutrient Content
3.5. Effect of OMF Product on Soil Water Retention
3.6. Plant Phytosanitary Properties
3.7. Nutrient Composition of Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OM | Organic matter |
| PUE | Phosphorus use efficiency |
| OMWS | Olive mill waste sludge |
| OMF | Organo-mineral fertilizers |
| RP | Rock phosphate |
| PWS | Phosphate washing sludge |
| SOM | Soil organic matter |
| FUE | Fertilizer use efficiency |
| AP | Available phosphorus |
| MSI | Membrane stability index |
| RWC | Relative water content |
| WUE | Water use efficiency |
| PAE | Phosphorus agronomic efficiency |
| PSR | Phosphorus solubilization rate |
References
- WPP. World Populations Prospects, Key Findings and Advance Table; United Nations: New York, NY, USA, 2017; Volume 1. [Google Scholar]
- Hoffland, E.; Kuyper, T.W.; Comans, R.N.J.; Creamer, R.E. Eco-Functionality of Organic Matter in Soils. Plant Soil 2020, 455, 1–22. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organisation of the United Nation). Agricultural Production Statistics 2000–2020; FAOSTAT Da. 2021. Available online: https://www.fao.org/3/cb9180en/cb9180en.pdf (accessed on 30 October 2022).
- Çokkizgin, A.; Girgel, Ü.; Kara, Z.; Çölkesen, M.; Saltali, K.; Yürürdurmaz, C. Organik Gübrelerin Mısır Bitkisinin Verim Bileşenleri Ile Tanenin Protein ve Nişasta Içeriğine Etkisi. Harran Tarım ve Gıda Bilim. Derg. 2022, 26, 133–142. [Google Scholar] [CrossRef]
- Obalum, S.E.; Chibuike, G.U.; Peth, S.; Ouyang, Y. Soil Organic Matter as Sole Indicator of Soil Degradation. Environ. Monit. Assess. 2017, 189, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Kenneth, G.C.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices. Nat. Publ. Gr. 2002, 122, 215. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Baghel, D.; Shukla, C.S.; Singh, H.K. Role of Different Substrates and Organic Supplements on Growth and Yield of Different Strains of Calocybe Indica. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2263–2269. [Google Scholar] [CrossRef]
- Hua, W.; Luo, P.; An, N.; Cai, F.; Zhang, S.; Chen, K.; Yang, J.; Han, X. Manure Application Increased Crop Yields by Promoting Nitrogen Use Efficiency in the Soils of 40-Year Soybean-Maize Rotation. Sci. Rep. 2020, 10, 14882. [Google Scholar] [CrossRef]
- Krishnaraj, P.U.; Dahale, S. Mineral Phosphate Solubilization: Concepts and Prospects in Sustainable Agriculture. Proc. Indian Natl. Sci. Acad. 2014, 80, 389–405. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [Green Version]
- Goucher, L.; Bruce, R.; Cameron, D.D.; Lenny Koh, S.C.; Horton, P. The Environmental Impact of Fertilizer Embodied in a Wheat-to-Bread Supply Chain. Nat. Plants 2017, 3, 17012. [Google Scholar] [CrossRef] [Green Version]
- Bouhia, Y.; Lyamlouli, K.; Ouhdouch, Y.; El Boukhari, M.E.M.; Hafidi, M. Agronomic Assessment of Solar Dried Recycled Olive Mill Sludge on Maize Agrophysiological Traits and Soil Fertility. Int. J. Recycl. Org. Waste Agric. 2022, 11, 247–261. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; El Boukhari, M.E.M.; Zeroual, Y.; Lyamlouli, K. Effect of the Co-Application of Olive Waste-Based Compost and Biochar on Soil Fertility and Zea Mays Agrophysiological Traits. Int. J. Recycl. Org. Waste Agric. 2021, 10, 111–127. [Google Scholar] [CrossRef]
- Rigane, H.; Chtourou, M.; Mahmoud, I.B.; Medhioub, K.; Ammar, E. Polyphenolic compounds progress during olive mill wastewater sludge and poultry manure co-composting, and humic substances building (Southeastern Tunisia). Waste Manag. Res. 2015, 33, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V. Agriculture, Ecosystems and Environment Physico-Chemical Properties and Microbial Responses in Biochar-Amended Soils: Mechanisms and Future Directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Semida, W.M. Organo mineral fertilizer can mitigate water stress for cucumber production (Cucumis sativus L.). Agric. Water Manag. 2015, 159, 1–10. [Google Scholar] [CrossRef]
- Sia, Z.Y.; Ch’ng, H.Y.; Liew, J.Y. Amending inorganic fertilizers with rice straw compost to improve soil nutrients availability, nutrients uptake, and dry matter production of maize (Zea mays L.) cultivated on a tropical acid soil. AIMS Agric. Food 2019, 4, 1020–1033. [Google Scholar] [CrossRef]
- Ayeni, L.; Ezeh, O.S. Comparative effect of NPK 20:10:10, organic and organo-mineral fertilizers on soil chemical properties, nutrient uptake and yield of tomato (Lycopersicon esculentum). Appl. Trop. Agric. 2017, 22, 111–116. [Google Scholar] [CrossRef]
- Antille, D.L.; Sakrabani, R.; Tyrrel, S.F.; Le, M.S.; Godwin, R.J. Development of Organomineral Fertilisers Derived from Nutrient-Enriched Biosolids Granules: Product Specification. Am. Soc. Agric. Biol. Eng. Annu. Int. Meet. 2013 2013, 5, 4152–4170. [Google Scholar] [CrossRef]
- Bakhashwain, A.A.; Daur, I.; Abohassan, R.A.A.; El-Nakhlawy, F.S. Response of Genetically Divergent Pearl Millet [Pennisetum Glaucum (L.) R. Br.] Varieties to Different Organo-Mineral Fertility Management. Pakistan J. Bot. 2013, 45, 1657–1661. [Google Scholar] [CrossRef] [Green Version]
- Farrar, M.B.; Wallace, H.M.; Xu, C.Y.; Nguyen, T.T.N.; Tavakkoli, E.; Joseph, S.; Bai, S.H. Short-Term Effects of Organo-Mineral Enriched Biochar Fertiliser on Ginger Yield and Nutrient Cycling. J. Soils Sediments 2018, 19, 668–682. [Google Scholar] [CrossRef]
- Ayeni, L.S. Effect of Combined Cocoa Pod Ash and NPK Fertilizer on Soil Properties, Nutrient Uptake and Yield of Maize (Zea Mays). J. Am. Folk. 2010, 6, 79–84. [Google Scholar]
- Makinde, E.A. Effects of an Organo–Mineral Fertilizer Application on the Growth and Yield of Maize. Aust. J. Basic Appl. Sci. 2007, 3, 15–19. [Google Scholar]
- Carvalho, R.P.; Moreira, R.A.; Cruz, M.C.M.; Fernandes, D.R.; Oliveira, A.F. Organomineral Fertilization on the Chemical Characteristics of Quartzarenic Neosol Cultivated with Olive Tree. Sci. Hortic. 2014, 176, 120–126. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of Organic Matter on Phosphorus Adsorption and Desorption in a Black Soil from Northeast China. Soil Tillage Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Weimin, G.; Chen, W.; Yang, F.; Zhang, L.; Wang, J. Organic Acid Secretion and Phosphate Solubilizing Efficiency of Pseudomonas sp. PSB12: Effects of Phosphorus Forms and Carbon Sources. J. Geomicrobiol. 2015, 33, 870–877. [Google Scholar] [CrossRef]
- Trupiano, D.; Cocozza, C.; Baronti, S.; Amendola, C.; Vaccari, F.P.; Lustrato, G.; Di Lonardo, S.; Fantasma, F.; Tognetti, R.; Scippa, G.S. The Effects of Biochar and Its Combination with Compost on Lettuce (Lactuca Sativa L.) Growth, Soil Properties, and Soil Microbial Activity and Abundance. Int. J. Agron. 2017, 2017, 315820. [Google Scholar] [CrossRef] [Green Version]
- Coyotl, M.D.L.A.V.; Tecpoyotl, Z.G.L.; Castro, E.S.; Campante, M.A.T.; Peralta, M.A.C. Theorgano-Mineral Fertilization on the Yield of Faba Bean in Soil and Hydroponics in Protected Agriculture. Rev. Mex. De Cienc. Agrícolas 2018, 9, 1603–1614. [Google Scholar]
- Hafidi, M.; Amir, S.; Revel, J. Structural Characterization of Olive Mill Waster-Water after Aerobic Digestion using Elemental analysis, FTIR and 13 C NMR. Process Biochem. 2005, 40, 2615–2622. [Google Scholar] [CrossRef]
- De Silva, C.S.; Koralage, I.S.A.; Weerasinghe, P.; Silva, N.R.N. The Determination of Available Phosphorus in Soil: A Quick and Simple Method. OUSL J. 2015, 8, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Moor, C.; Lymberopoulou, T.; Dietrich, V.J. Determination of Heavy Metals in Soils, Sediments and Geological Materials by ICP-AES and ICP-MS. Mikrochim. Acta 2001, 136, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Xu, H.; Zhou, P.; Tan, Y. Investigation of Aggregate Moisture Content Variation and Its Impact on Pavement Performance of WMA. Constr. Build. Mater. 2020, 255, 119350. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Rady, M.M. A Novel Organo-Mineral Fertilizer Can Mitigate Salinity Stress Effects for Tomato Production on Reclaimed Saline Soil. S. Afr. J. Bot. 2012, 81, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Ali, B.; Aiman Hasan, S.; Ahmad, A. Brassinosteroid Enhanced the Level of Antioxidants under Cadmium Stress in Brassica Juncea. Environ. Exp. Bot. 2007, 60, 33–41. [Google Scholar] [CrossRef]
- Kapellakis, I.; Tzanakakis, V.A.; Angelakis, A.N. Land Application-Based Olive Mill Wastewater Μanagement. Water 2015, 7, 362–376. [Google Scholar] [CrossRef] [Green Version]
- Dobermann, A. Fertilizer Best Management Practices (FBMP’s); International Fertilizer Industry Association: Paris, France, 2007; ISBN 295231392X. [Google Scholar]
- Ditta, A.; Muhammad, J.; Imtiaz, M.; Mehmood, S.; Qian, Z.; Tu, S. Application of Rock Phosphate Enriched Composts Increases Nodulation, Growth and Yield of Chickpea. Int. J. Recycl. Org. Waste Agric. 2018, 7, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Hu, Z.; Wan, Q.; Mu, B.; Li, G.; Yang, Y. Integrated Application of Inorganic and Organic Fertilizer Enhances Soil Organo-Mineral Associations and Nutrients in Tea Garden Soil. Agronomy 2022, 12, 1330. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Growth, Yield, Fruit Quality and Nutrient Uptake of Hydroponically Cultivated Zucchini Squash as Affected by Irrigation Systems and Growing Seasons. Sci. Hortic. 2005, 105, 177–195. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Ludlow, M.M. Influence of Soil Water Supply on the Plant Water Balance of Four Tropical Grain Legumes. Aust. J. Plant Physiol. 1986, 13, 329–341. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Boukhari, M.E.M.; Mphatso, C.; Zeroual, Y.; Lyamlouli, K. Conversion of Waste into Organo-Mineral Fertilizers: Current Technological Trends and Prospects. Rev. Environ. Sci. Biotechnol. 2022, 21, 425–446. [Google Scholar] [CrossRef]
- Liang, J.; Yang, Z.; Tang, L.; Zeng, G.; Yu, M.; Li, X.; Wu, H.; Qian, Y.; Li, X.; Luo, Y. Chemosphere Changes in Heavy Metal Mobility and Availability from Contaminated Wetland Soil Remediated with Combined Biochar-Compost. Chemosphere 2017, 181, 281–288. [Google Scholar] [CrossRef]
- Tsai, C.; Chang, Y.-F. Carbon Dynamics and Fertility in Biochar-Amended Soils with Excessive Compost Application. Agronomy 2019, 9, 511. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, N.; Saigusa, M.; Sakaiya, E.; Tamakawa, K.; Oyamada, Z.; Kudo, K. Acidification and Soil Productivity of Allophanic Andosols Affected by Heavy Application of Fertilizers. Soil Sci. Plant Nutr. 2005, 1, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Disciglio, G.; Carlucci, A.; Tarantino, A.; Giuliani, M.M.; Gagliardi, A.; Frabboni, L.; Libutti, A.; Raimondo, M.L.; Lops, F.; Gatta, G. Effect of Olive-Mill Wastewater Application, Organo-Mineral Fertilization, and Transplanting Date on the Control of Phelipanche Ramosa in Open-Field Processing Tomato Crops. Agronomy 2018, 8, 92. [Google Scholar] [CrossRef]
- Bonanomi, G.; Ippolito, F.; Cesarano, G.; Nanni, B.; Lombardi, N.; Rita, A.; Saracino, A.; Scala, F. Biochar As Plant Growth Promoter: Better Off Alone or Mixed with Organic Amendments. Front. Plant Sci. 2017, 8, 1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deluca, T.H.; Derek, M.M.; Jones, D.L. Biochar Effects on Soil Nutrient Transformations. Biochar Environ. Manag. Routledge 2015, 2, 420–452. [Google Scholar]
- Saleem, H.; Rehman, K.; Arslan, M.; Afzal, M. Enhanced Degradation of Phenol in Floating Treatment Wetlands by Plant-Bacterial Synergism. Int. J. Phytoremediation 2018, 20, 692–698. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; El Boukhari, M.E.M.; El Fels, L.; Zeroual, Y.; Lyamlouli, K. Microbial Community Succession and Organic Pollutants Removal During Olive Mill Waste Sludge and Green Waste Co-Composting. Front. Microbiol. 2022, 12, 4385. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Kulikowska, D. Behaviors of Heavy Metals (Cd, Cu, Ni, Pb and Zn) in Soil Amended with Composts. Environ. Technol. 2016, 37, 2337–2347. [Google Scholar] [CrossRef]
- Fan, C.; Du, B.; Zhang, Y.; Ding, S.; Gao, Y.; Chang, M. Adsorption of Lead on Organo-Mineral Complexes Isolated from Loess in Northwestern China. J. Geochem. Explor. 2017, 176, 50–56. [Google Scholar] [CrossRef]
- Gundale, M.J.; Deluca, T.H. Temperature and Source Material Influence Ecological Attributes of Ponderosa Pine and Douglas-Fir Charcoal. For. Ecol. Manag. 2006, 231, 86–93. [Google Scholar] [CrossRef]
- Sigua, G.C.; Novak, J.M.; Watts, D.W.; Johnson, M.G.; Spokas, K. Chemosphere Efficacies of Designer Biochars in Improving Biomass and Nutrient Uptake of Winter Wheat Grown in a Hard Setting Subsoil Layer. Chemosphere 2016, 142, 176–183. [Google Scholar] [CrossRef]
- Bouhia, Y.; Lyamlouli, K.; El Fels, L.; Youssef, Z.; Ouhdouch, Y.; Hafidi, M. Effect of Microbial Inoculation on Lipid and Phenols Removal During the Co-Composting of Olive Mill Solid Sludge with Green Waste in Bioreactor. Waste Biomass Valorization 2021, 12, 1417–1429. [Google Scholar] [CrossRef]
- Bohme, L.; Langer, U.; Bohme, F. Microbial Biomass, Enzyme Activities and Microbial Community Structure in Two European Long-Term Field Experiments. Agric. Ecosyst. Environ. 2005, 109, 141–152. [Google Scholar] [CrossRef]
- Masto, R.E.; Kumar, S.; Rout, T.K.; Sarkar, P.; George, J.; Ram, L.C. Catena Biochar from Water Hyacinth (Eichornia Crassipes) and Its Impact on Soil Biological Activity. Catena 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Chandra, R.; Singh, S.; Reddy, M.M.K.; Patel, D.K.; Purohit, H.J.; Kapley, A. Isolation and Characterization of Bacterial Strains Paenibacillus Sp. and Bacillus Sp. for Kraft Lignin Decolorization from Pulp Paper Mill Waste. J. Gen. Appl. Microbiol. 2008, 54, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Ding, W.; Zhang, J.; Li, Y.; Luo, J.; Bolan, N.; Xie, Z. Soil Biology & Biochemistry Biochar Suppressed the Decomposition of Organic Carbon in a Cultivated Sandy Loam Soil: A Negative Priming Effect. Soil Biol. Biochem. 2014, 76, 12–21. [Google Scholar] [CrossRef]
- Prommer, J.; Wanek, W.; Hofhansl, F.; Trojan, D.; Offre, P.; Urich, T.; Schleper, C.; Sassmann, S.; Kitzler, B.; Soja, G.; et al. Biochar Decelerates Soil Organic Nitrogen Cycling but Stimulates Soil Nitrification in a Temperate Arable Field Trial. PLoS ONE 2014, 9, e86388. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential Mechanisms for Achieving Agricultural Benefits from Biochar Application to Temperate Soils: A Review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Blackwell, A.P.; Krull, B.E.; Butler, C.G.; Herbert, A.A.; Solaiman, D.Z. Effect of Banded Biochar on Dryland Wheat Production and Fertiliser Use in South-Western Australia: An Agronomic and Economic Perspective. Soil Res. 2010, 48, 531–545. [Google Scholar] [CrossRef]
- Ruiz-lozano, J.M.; Porcel, R.; Bárzana, G.; Azcón, R. Contribution of Arbuscular Mycorrhizal Symbiosis to Plant Drought Tolerance: State of the Art. Plant Responses Drought Stress 2012, 13, 335–361. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using Poultry Litter Biochars as Soil Amendments. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H. Reconciling Apparent Variability in Effects of Biochar Amendment on Soil Enzyme Activities by Assay Optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Burns, R.G.; Deforest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil Biology & Biochemistry Soil Enzymes in a Changing Environment: Current Knowledge and Future Directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Qiao, Y.; Miao, S.; Zhong, X.; Zhao, H.; Pan, S. The Greatest Potential Benefit of Biochar Return on Bacterial Community Structure among Three Maize-Straw Products after Eight-Year Field Experiment in Mollisols. Appl. Soil Ecol. 2020, 147, 103432. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Wang, F.; Tang, J.; Yu, L.; Zhang, R. Effects of Biochar Amendment on Soil Aggregates and Hydraulic Properties. J. Soil Sci. Plant Nutr. 2013, 13, 991–1002. [Google Scholar] [CrossRef] [Green Version]
- Ameen, A.; Ahmad, J.; Raza, S. Determination of CEC to Evaluate the Quality and Maturity of Compost Prepared by Using Municipal Solid Waste. Int. J. Sci. Res. Publ. 2016, 6, 42–44. [Google Scholar]
- Ojeda, G.; Mattana, S.; Àvila, A.; Alcañiz, J.M.; Volkmann, M.; Bachmann, J. Are Soil-Water Functions Affected by Biochar Application? Geoderma 2015, 249–250, 1–11. [Google Scholar] [CrossRef]
- Liyanage, T.D.P.; Leelamanie, D.A.L. Influence of Organic Manure Amendments on Water Repellency, Water Entry Value, and Water Retention of Soil Samples from a Tropical Ultisol. J. Hydrol. Hydromech. 2016, 64, 160. [Google Scholar] [CrossRef] [Green Version]
- Minasny, B.; Mcbratney, A.B. Integral Energy as a Measure of Soil-Water Availability. Plant Soil 2003, 249, 253–262. [Google Scholar] [CrossRef]
- Miller, V.S.; Naeth, M.A. Hydrogel and Organic Amendments to Increase Water Retention in Anthroposols for Land Reclamation. Appl. Environ. Soil Sci. 2019, 2019, 4768091. [Google Scholar] [CrossRef]
- Abedi-Koupai, J.; Sohrab, F.; Swarbrick, G. Evaluation of Hydrogel Application on Soil Water Retention Characteristics. J. Plant Nutr. 2008, 31, 317–331. [Google Scholar] [CrossRef]
- Benavente, I.; Gascó, G.; Plaza, C.; Paz-Ferreiro, J.; Méndez, A. Choice of Pyrolysis Parameters for Urban Wastes Affects Soil Enzymes and Plant Germination in a Mediterranean Soil. Sci. Total Environ. 2018, 634, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Dergacheva, M.I.; Nekrasova, O.A.; Okoneshnikova, M.V.; Vasil’eva, D.I.; Gavrilov, D.A.; Ochur, K.O.; Ondar, E.E. Ratio of Elements in Humic Acids as a Source of Information on the Environment of Soil Formation. Contemp. Probl. Ecol. 2012, 5, 497–504. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero MA, S.T. Physical and Chemical Characterization of Biochars Derived from Different Agricultural Residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Ahmed, M.; Ahmad, S.; Fayyaz-ul-Hassan; Qadir, G.; Hayat, R.; Shaheen, F.A.; Raza, M.A. Innovative Processes and Technologies for Nutrient Recovery from Wastes: A Comprehensive Review. Sustainability 2019, 11, 4938. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Zhang, Y.; Shao, H.; Sun, J. Science of the Total Environment Pyrolysis Temperature Affects Phosphorus Transformation in Biochar: Chemical Fractionation and 31 P NMR Analysis. Sci. Total Environ. 2016, 569–570, 65–72. [Google Scholar] [CrossRef]
- Schmidt:, H.P.; Kammann, C.; Niggli, C.; Evangelou, M.W.H.; Mackie, K.A.; Abiven, S. Biochar and Biochar-Compost as Soil Amendments to a Vineyard Soil: Influences on Plant Growth, Nutrient Uptake, Plant Health and Grape Quality. Agric. Ecosyst. Environ. 2014, 191, 117–123. [Google Scholar] [CrossRef]




| Parameters | pH | EC (ms/cm) | TOC (%) (mg kg−1) | TKN (%) (mg kg−1) | Total P2O5 (mg kg−1) | Total K2O (mg kg−1) | Total CaO g/kg | Total MgO (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
| Average | 8.2 | 0.38 | 0.7 | 0.87 | 50.3 | 325.7 | 12.1 | 646.0 |
| Treatment | EC (ms/m) | OM (%) | Available Phosphorus (mg kg−1) | Exch K2O (mg kg−1) | Exch MgO (mg/kg) |
|---|---|---|---|---|---|
| Control | 0.39 ± 0.1 ab | 1.2 ± 0.06 a | 50.3 ± 1.5 abc | 325.67 ± 59.04 a | 646.0 ± 18.6 a |
| OMF0 + DAP | 0.39 ± 0.19 ab | 1.21 ± 0.1 a | 60.3 ± 15.9 abcde | 345.0 ± 8.54 ab | 650.7 ± 23.7 a |
| OMF0 + RP | 0.3 ± 0.06 a | 1.16 ± 0.01 a | 49.0 ± 3.6 abc | 285.3 ± 11.0 a | 668.7 ± 16.2 ab |
| OMF0 + PWS | 0.3 ± 0.03 a | 1.18 ± 0.06 a | 52.0 ± 5.3 abc | 300.6 ± 19.3 a | 649.7 ± 2.2 a |
| OMF1 | 0.69 ± 0.1 abc | 1.43 ± 0.08 a | 43.0 ± 1.73 a | 366.0 ± 39.8 abc | 733.0 ± 47.3 abc |
| OMF1 + DAP | 0.77 ± 0.1 abc | 1.42 ± 0.04 a | 56.6 ± 6.6 abcd | 399.6 ± 23.6 abcd | 721.3 ± 94.4 abc |
| OMF1 + RP | 0.74 ± 0.18 abc | 1.47 ± 0.1 a | 43.3 ± 5.13 a | 387.6 ± 34.9 abcd | 747.0 ± 54 abc |
| OMF1 + PWS | 0.82 ± 0.19 abc | 1.37 ± 0.2 a | 47.6 ± 9.1 ab | 380.6 ± 23.6 abcd | 740.7 ± 34.4 abc |
| OMF2 | 0.98 ± 0.18 c | 2.37 ± 0.03 b | 68.0 ± 12.1 abcdef | 572.0 ± 62.23 d | 794.3 ± 35.6 abc |
| OMF2 + DAP | 0.98 ± 0.17 c | 2.39 ± 0.28 b | 85.0 ± 4.4 efg | 567.6 ± 30.6 d | 785.3 ± 94.4 abc |
| OMF2 + RP | 0.91 ± 0.25 bc | 2.33 ± 0.07 b | 70.3 ± 9.07 bcdef | 523.0 ± 153.1 bcd | 737.0 ± 54 abc |
| OMF2 + PWS | 0.9 ± 0.04 bc | 2.4 ± 0.04 b | 81.7 ± 10.2 defg | 548.7 ± 45.8 cd | 766.7 ± 34.4 abc |
| OMF3 | 2.8 ± 0.26 d | 2.9 ± 0.08 c | 73.6 ±6.35 cdefg | 794.3 ± 88.79 e | 808.3± 54.4 bc |
| OMF3 + DAP | 2.7 ± 0.24 d | 3.0 ± 0.17 c | 98.3 ± 6.66 g | 837.3 ± 11.01 e | 838.0± 42.6 c |
| OMF3 + RP | 2.9 ± 0.26 d | 2.98 ± 0.01 c | 80.3 ± 11.6 defg | 813.3 ± 69.3 e | 867.0± 67.3 c |
| OMF3 + PWS | 2.7 ± 0.1 d | 2.92 ± 0.06 c | 88.7 ± 8.6 fg | 795.3 ± 114.6 e | 831.0± 88.6 c |
| Treatment | EC (ms/m) | OM (%) | Available Phosphorus (mg kg−1) | Exch K2O (mg kg−1) | Exch MgO (mg/kg) |
|---|---|---|---|---|---|
| Control | 0.86 ± 0.1 ab | 1.16 ± 0.03 ab | 28.6 ± 3.22 abc | 233.6 ± 28.53 a | 723.3 ± 89.7 a |
| OMF0 + DAP | 0.87 ± 0.5 ab | 1.18 ± 0.1 ab | 38.0 ± 8.7 abcde | 239.3 ± 36.17 a | 769.0 ± 55.7 ab |
| OMF0 + RP | 0.7 ± 0.1 a | 1.06 ± 0.05 a | 27.0 ± 3.5 ab | 267.3 ± 5.589 a | 770.0 ± 27.4 ab |
| OMF0 + PWS | 0.92 ± 0.18 ab | 1.09 ± 0.06 a | 26.3 ± 3.06 ab | 273.3 ± 13.05 a | 965.6 ± 54.8 cde |
| OMF1 | 0.98 ± 0.1 ab | 1.29 ± 0.07 ab | 35.6 ± 5.7 abcde | 283.6 ± 17.6 a | 927.0 ± 58.14 bcd |
| OMF1 + DAP | 0.97 ± 0.3 ab | 1.22 ± 0.12 ab | 40.6 ± 13.01 abcde | 300.3 ± 40.51 a | 988.0 ± 82.2 cde |
| OMF1 + RP | 1.4 ± 0.2 abc | 1.24 ± 0.3 ab | 24.3 ± 5.03 a | 286.6 ± 34.2 a | 907.3 ± 93.8 abc |
| OMF1 + PWS | 1.12 ± 0.3 abc | 1.2 ± 0.13 ab | 32.6 ± 6.6 abcd | 332.3 ± 51 ab | 951.6 ± 42.8 bcde |
| OMF2 | 1.04 ± 0.1 ab | 1.5 ± 0.04 abc | 41.0 ± 1.7 abcde | 455.0 ± 68.6 bc | 922 ± 38.4 bcd |
| OMF2 + DAP | 1.13 ± 0.1 abc | 2.0 ± 0.28 cde | 65.0 ± 1.7 fg | 486.6 ± 65.8 c | 1007 ± 51.3 cde |
| OMF2 + RP | 1.4 ± 0.1 abc | 1.81 ± 0.2 bcd | 40.6 ± 8.5 abcde | 479.3 ± 51.3 bc | 1088 ± 93.1 cde |
| OMF2 + PWS | 1.5 ± 0.29 bc | 1.67 ± 0.5 abc | 50.0 ± 12.5 def | 503.3 ± 46.75 c | 1090 ± 86.6 cde |
| OMF3 | 1.41 ± 0.2 abc | 2.37 ± 0.32 de | 45.6 ± 3.5 bcdef | 851.3 ± 71.14 e | 1107 ± 55.2 de |
| OMF3 + DAP | 1.5 ± 0.4 bc | 2.5 ± 0.28 e | 76.0 ± 10.6 g | 842.6 ± 29.9 e | 1123 ± 55.2 e |
| OMF3 + RP | 1.8 ± 0.04 c | 2.5 ± 0.13 e | 56.0 ± 5 efg | 874.0 ± 102.1 e | 1320 ± 27.19 f |
| OMF3 + PWS | 1.6 ± 0.13 bc | 2.5 ± 0.26 e | 49.6 ± 5.03 cdef | 659.6 ± 21.1 d | 1012 ± 1 cde |
| Treatment | MSI (%) | RWC (%) | Ear Yield (g dry Matter) | Leaf Number Per Plant | WUE (kg m−3) |
|---|---|---|---|---|---|
| Control | 40.3 ± 0.6 a | 65 ± 2.5 a | 8.19 ± 0.9 ab | 8 ± 0.8 ab | 3.94 ± 2.3 ab |
| OMF0 + DAP | 42.7 ± 2.2 abc | 70.3 ± 1.05 ab | 21.04 ± 1.7 de | 11.3 ± 0.58 cdef | 10.1 ± 0.81 abc |
| OMF0 + RP | 40.9 ± 1.6 a | 67.13 ± 2.8 ab | 10.33 ± 0.98 abc | 7.7 ± 0.96 a | 4.96 ± 4.2 ab |
| OMF0 + PWS | 41.2 ± 1.92 ab | 67.26 ± 2.7 ab | 7.69 ± 0.33 a | 10.2 ± 0.95 abcde | 3.7 ± 1.4 a |
| OMF1 | 40.7 ± 3.87 a | 68.58 ± 1.02 ab | 28.9 ± 8.7 e | 8.2 ± 0.5 ab | 13.9 ± 3.5 abc |
| OMF1 + DAP | 42.7 ± 0.83 abc | 73.2 ± 0.67 b | 13.91 ± 0.9 abcd | 13.4 ± 2.4 f | 6.68 ± 2.2 abc |
| OMF1 + RP | 53.2 ± 1.1 f | 71.5 ± 1.4 ab | 7.7 ± 1.05 a | 8.5 ± 1.2 abc | 3.7 ± 0.5 ab |
| OMF1 + PWS | 46.16 ± 2.8 bcd | 73.1 ± 1.9 b | 7.69 ± 1.2 a | 9.2 ± 0.5 abcd | 3.69 ± 1.4 abc |
| OMF2 | 47.9 ± 1.35 de | 73.4 ± 2.6 b | 9.4 ± 1.2 abc | 12.4 ± 0.6 ef | 4.5 ± 3.56 ab |
| OMF2 + DAP | 41.3 ± 0.67 abc | 69.9 ± 3.3 ab | 16.5 ± 2.9 bcd | 12 ± 1 def | 7.81 ± 6.1 abc |
| OMF2 + RP | 44.3 ± 0.6 abcd | 68.4 ± 2.57 ab | 13.5 ± 3.2 abcd | 8.75 ± 0.5 abc | 6.47 ± 1.18 abc |
| OMF2 + PWS | 46.4 ± 1.7 cd | 71.48 ± 3.1 ab | 14.9 ± 2.4 abcd | 11.2 ± 0.5 cdef | 7.2 ± 4.08 abc |
| OMF3 | 52.9 ± 0.2 ef | 72.6 ± 2.57 b | 17.6 ± 1.8 cd | 9.5 ± 1 abcde | 8.45 ± 2.43 abc |
| OMF3 + DAP | 53.7 ± 0.5 f | 72.9 ± 3.02 b | 21.7 ± 1.9 de | 10.7 ± 1.7 bcdef | 10.45 ± 3.6 abc |
| OMF3 + RP | 42.9 ± 1.7 abcd | 71.4 ± 0.6 ab | 19.4 ± 2.6 d | 12.2 ± 1.5 ef | 9.31 ± 0.78 abc |
| OMF3 + PWS | 43.3 ± 0.7 abcd | 69.4 ± 3.7 ab | 21.1 ± 2.2 de | 13.7 ± 0.6 f | 11.3 ± 1.2 bc |
| Treatment | N% | P% | K% | Mg% | Ca% | Zn mg/kg |
|---|---|---|---|---|---|---|
| Control | 0.346 ± 0.006 a | 0.07 ± 0.01 a | 2.04 ± 0.12 abc | 0.3 ± 0.05 bc | 0.7 ± 0.02 bc | 18.8 ± 6.09 a |
| OMF0 + DAP | 0.343 ± 0.05 a | 0.11 ± 0.1 abcde | 2.087 ± 0.08 abcd | 0.29 ± 0.04 bc | 0.6 ± 0.01 a | 20.37 ± 1.14 ab |
| OMF0 + RP | 0.34 a | 0.083 ± 0.01 ab | 1.9 ± 0.7 a | 0.34 ± 0.02 bcd | 0.5 ± 0.02 a | 23.1 ± 5.41 ab |
| OMF0 + PWS | 0.34 ± 0.017 a | 0.087 abc | 2.02 ± 0.073 ab | 0.2 ± 0.01 a | 0.6 ± 0.035 a | 17.6 ± 1.27 a |
| OMF1 | 0.386 ± 0.011 ab | 0.083 ± 0.02 ab | 2.3 ± 0.14 abcde | 0.36 ± 0.03 cd | 0.74 ± 0.16 c | 21.3 ± 2.39 ab |
| OMF1 + DAP | 0.38 ± 0.01 ab | 0.13 ± 0.01 bcdefg | 2.2 ± 0.12 abcd | 0.29 ± 0.01 bc | 0.58 ± 0.03 a | 20.6 ± 2.2 ab |
| OMF1 + RP | 0.37 ± 0.027 ab | 0.09 ± 0.005 abcd | 1.9 ± 0.08 a | 0.32 ± 0.01 bcd | 0.59 ± 0.02 a | 19.9 ± 7.36 ab |
| OMF1 + PWS | 0.38 ± 0.01 ab | 0.11 ± 0.005 abcdef | 2.02 ± 0.19 ab | 0.276 ± 0.03 ab | 0.62 ± 0.06 a | 19.9 ± 2.2 ab |
| OMF2 | 0.42 ± 0.015 ab | 0.137 ± 0.02 bcdefg | 2.47 ± 0.05 cde | 0.32 ± 0.01 bcd | 0.65 ± 0.03 a | 25.7 ± 7.36 ab |
| OMF2 + DAP | 0.46 ± 0.05 b | 0.187 ± 0.015 gh | 2.79 ± 0.12 f | 0.40 ± 0.03 d | 0.57 ± 0.03 a | 33.7 ± 2.7 b |
| OMF2 + RP | 0.43 ± 0.068 ab | 0.17 ± 0.01 fgh | 2.18 ± 0.2 abcd | 0.36 ± 0.02 cd | 0.55 ± 0.04 ab | 27.6 ± 8 ab |
| OMF2 + PWS | 0.38 ± 0.01 ab | 0.14 ± 0.016 cdefgh | 2.45 ± 0.3 cdef | 0.35 ± 0.01 bcd | 0.71 ± 0.08 bc | 23.7 ± 5.14 ab |
| OMF3 | 0.456 ± 0.05 b | 0.14 ± 0.02 cdefgh | 2.47 ± 0.16 def | 0.32 ± 0.01 bc | 0.6 ± 0.03 a | 33.7 ± 2.7 b |
| OMF3 + DAP | 0.41 ± 0.02 ab | 0.19 ± 0.04 h | 2.7 ± 0.08 ef | 0.40 ± 0.06 d | 0.5 ± 0.026 a | 27.59 ± 8.2 ab |
| OMF3 + RP | 0.43 ± 0.08 ab | 0.17 ± 0.01 efgh | 2.1 ± 0.053 abcd | 0.36 ± 0.03 cd | 0.5 ± 0.09 a | 26.36 ± 1.06 ab |
| OMF3 + PWS | 0.39 ± 0.02 ab | 0.14 ± 0.04 defg | 2.4 ± 0.14 bcdef | 0.35 ± 0.03 bcd | 0.7 ± 0.1 bc | 17.62 ± 4.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Zeroual, Y.; Lyamlouli, K. Organo-Mineral Fertilization Based on Olive Waste Sludge Compost and Various Phosphate Sources Improves Phosphorus Agronomic Efficiency, Zea mays Agro-Physiological Traits, and Water Availability. Agronomy 2023, 13, 249. https://doi.org/10.3390/agronomy13010249
Bouhia Y, Hafidi M, Ouhdouch Y, Zeroual Y, Lyamlouli K. Organo-Mineral Fertilization Based on Olive Waste Sludge Compost and Various Phosphate Sources Improves Phosphorus Agronomic Efficiency, Zea mays Agro-Physiological Traits, and Water Availability. Agronomy. 2023; 13(1):249. https://doi.org/10.3390/agronomy13010249
Chicago/Turabian StyleBouhia, Youness, Mohamed Hafidi, Yedir Ouhdouch, Youssef Zeroual, and Karim Lyamlouli. 2023. "Organo-Mineral Fertilization Based on Olive Waste Sludge Compost and Various Phosphate Sources Improves Phosphorus Agronomic Efficiency, Zea mays Agro-Physiological Traits, and Water Availability" Agronomy 13, no. 1: 249. https://doi.org/10.3390/agronomy13010249