A Composite Bioinoculant Based on the Combined Application of Beneficial Bacteria and Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Examined Strains
2.2. Determination of Biocontrol Index (BCI) Values
2.3. Liquid and Solid-State Fermentations
2.4. Enzyme Activity Measurements
2.5. Growth Assay in Nitrogen-Free Medium
2.6. Plant Material for Growth Chamber Experiments
2.7. Measurement of Stomatal Conductance, CO2 Assimilation, and Total Soluble Sugar Content
2.8. Chlorophyll a Fluorescence Measurements
2.9. Pigment Analysis
2.10. Field Experiment
2.11. Soil Examination Methods
2.12. Statistical Analysis
3. Results
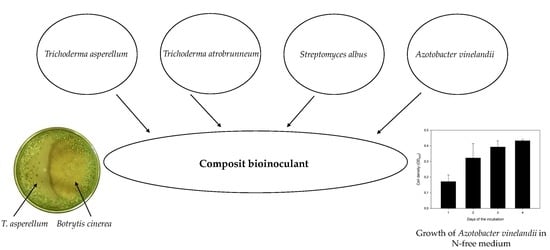
3.1. Selection of the Components for the Soil Inoculant
3.2. Influence of T. asperellum Strain SZMC 20786 on the Shoot and Root Growth and Photosynthetic Activity of Tomato Plants
3.3. Solid State Fermentation of Plant Stem Residues with T. atrobrunneum in Comparison with T. reesei
3.4. Field Experiment with the Combination of the Selected Bioinoculant Strains in Tomato Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, T.; Stevens, C.J.; Duchateau, L.; Jacquemyn, H.; Gowing, D.J.; Merckx, R.; Wallace, H.; van Rooijen, N.; Goethem, T.; Bobbink, R.; et al. Soil phosphorus constrains biodiversity across European grasslands. Glob. Chang. Biol. 2014, 20, 3814–3822. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, G.S. Fertilizer-N use efficiency and nitrate pollution of groundwater in developing countries. J. Contam. Hydrol. 1995, 20, 167–184. [Google Scholar]
- Meyer, S.L.F.; Roberts, D.P. Combinations of biocontrol agents for management of plant-parasitic nematodes and soilborne plant-pathogenic fungi. J. Nematol. 2002, 34, 1. [Google Scholar] [PubMed]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, D.M.; Thomashow, L.S.; Cook, R.J. Biological control of soil-borne pathogens of wheat: Benefits, risks and current challenges. In Biological Control: Benefits and Risks; Hokkanen, H.M.T., Lynch, J.M., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 149–160. [Google Scholar]
- Körmöczi, P.; Danilovic, G.; Manczinger, L.; Jovanovic, L.; Pankovic, D.; Vágvölgyi, C.; Kredics, L. Species composition of Trichoderma isolates from the rhizosphere of vegetables grown in Hungarian soils. Fresen. Environ. Bull. 2013, 22, 1736–1741. [Google Scholar]
- Javaid, A. Effects of biofertilizers combined with different soil amendments on potted rice plants. Chilean J. Agric. Res. 2011, 71, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Bóka, B.; Kedves, O.; Nagy, V.D.; Szűcs, A.; Champramary, S.; Roszik, R.; Patocskai, Z.; Münsterkötter, M.; Huynh, T.; et al. Towards the biological control of devastating forest pathogens from the genus Armillaria. Forests 2019, 10, 1013. [Google Scholar] [CrossRef] [Green Version]
- Szekeres, A.; Leitgeb, B.; Kredics, L.; Manczinger, L.; Vágvölgyi, C. A novel, image analysis-based method for the evaluation of in vitro antagonism. J. Microbiol. Meth. 2006, 65, 619–622. [Google Scholar] [CrossRef]
- Poór, P.; Gémes, K.; Horváth, F.; Szepesi, Á.; Simon, M.L.; Tari, I. Salicylic acid treatment via the rooting medium interferes with stomatal response, CO2 fixation rate and carbohydrate metabolism in tomato, and decreases harmful effects of subsequent salt stress. Plant Biol. 2011, 13, 105–114. [Google Scholar]
- Dubois, M.; Gibbs, K.A.; Hamilton, J.K.; Roberts, D.A.; Smith, F. Colorimetric methods for the determination of sugars and related substances. Anal. Chem. 1956, 28, 350–352. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Schreiber, U. Energy-dependent quenching of dark level chlorophyll fluorescence in intact leaves. Photosynth. Res. 1986, 10, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Ábrahám, B.É.; Őri, N.; Szabó, S.; Blaskó, L.; Zsigrai, G.Y. Effects of whitening process on the quality of different grain sorghum hybrids. Eur. J. Plant Sci. Biotechnol. 2012, 6, 90–93. [Google Scholar]
- Szolnoki, Z.; Farsang, A.; Puskás, I. Cumulative impacts of human activities on urban garden soils: Origin and accumulation of metals. Environ. Pollut. 2013, 177, 106–115. [Google Scholar] [CrossRef]
- Stefanovits, P.; Filep, G.; Füleky, G. Talajtan; Mezőgazda Kiadó: Budapest, Hungary, 1999; pp. 470p. (In Hungarian) [Google Scholar]
- Junsomboon, J.; Jakmunee, J. Determination of potassium, sodium and total alkalies in Portland cement, fly ash, admixtures, and water of concrete by a simple flow injection flame photometric system. J. Autom. Meth. Manag. Chem. 2011, 2011, 742656. [Google Scholar] [CrossRef]
- Kredics, L.; Hatvani, L.; Naeimi, S.; Körmöczi, P.; Manczinger, L.; Druzhinina, I.S. Biodiversity of the genus Hypocrea/Trichoderma in different habitats. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2014; pp. 3–24. [Google Scholar]
- Olmedo Monfil, V.; Casas-Flores, S. Molecular mechanisms of biocontrol in Trichoderma spp. and their applications in agriculture. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2014; pp. 429–454. [Google Scholar]
- Stewart, A.; Hill, R. Applications of Trichoderma in plant growth promotion. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2014; pp. 415–428. [Google Scholar]
- Saldajeno, M.G.B.; Naznin, H.A.; Elsharkawy, M.M.; Shimizu, M.; Hyakumachi, M. Enhanced resistance of plants to disease using Trichoderma spp. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2014; pp. 477–494. [Google Scholar]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J.S.; López-Bucio, J. Enhanced plant immunity using Trichoderma. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2014; pp. 495–504. [Google Scholar]
- Zaidi, N.W.; Dar, M.H.; Singh, S.; Singh, U.S. Trichoderma species as abiotic stress relievers in plants. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2014; pp. 515–526. [Google Scholar]
- Hatvani, L.; Kocsubé, S.; Manczinger, L.; Antal, Z.; Szekeres, A.; Druzhinina, I.S.; Komoń-Zelazowska, M.; Kubicek, C.P.; Nagy, A.; Vágvölgyi, C.; et al. The green mould disease global threat to the cultivation of oyster mushroom (Pleurotus ostreatus): A review. In Science and Cultivation of Edible and Medicinal Fungi: Mushroom Science XVII: Proceedings of the 17th Congress of the International Society for Mushroom Science; Gruening, M., Ed.; International Society for Mushroom Science: Cape Town, South Africa, 2008; pp. 485–495. [Google Scholar]
- Kredics, L.; García Jimenez, L.; Naeimi, S.; Czifra, D.; Urbán, P.; Manczinger, L.; Vágvölgyi, C.; Hatvani, L. A challenge to mushroom growers: The green mould disease of cultivated champignons. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; FORMATEX: Badajoz, Spain, 2010; pp. 295–305. [Google Scholar]
- Błaszczyk, L.; Siwulski, M.; Sobieralski, K.; Fruzynska-Jozwiak, D. Diversity of Trichoderma spp. causing Pleurotus green mould diseases in Central Europe. Folia Microbiol. 2013, 58, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Hatvani, L.; Manczinger, L.; Vágvölgyi, C.; Kredics, L. Trichoderma as a human pathogen. In Trichoderma-Biology and Applications; Mukherjee, P.K., Horwitz, B.A., Singh, U.S., Mukherjee, M., Schmoll, M., Eds.; CAB International: Wallingford, UK, 2013; pp. 292–313. [Google Scholar]
- Viterbo, A.; Landau, U.; Kim, S.; Chernin, L.; Chet, I. Characterization of ACC deaminase from the biocontrol and plant growth-promoting agent Trichoderma asperellum T203. FEMS Microbiol. Lett. 2010, 305, 42–48. [Google Scholar] [CrossRef]
- Brotman, Y.; Lisec, J.; Méret, M.; Chet, I.; Willmitzer, L.; Viterbo, A. Transcript and metabolite analysis of the Trichoderma-induced systemic resistance response to Pseudomonas syringae in Arabidopsis thaliana. Microbiology 2012, 158, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, W.; Zhao, L. Study of the siderophore-producing Trichoderma asperellum Q1 on cucumber growth promotion under salt stress. J. Basic Microbiol. 2013, 53, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Doni, F.; Isahak, A.; Zain, C.R.C.M.; Yusoff, W.M.W. Physiological and growth response of rice plants (Oryza sativa L.) to Trichoderma spp. inoculants. AMB Exp. 2014, 4, 45. [Google Scholar] [CrossRef]
- Azarmi, R.; Hajieghrari, B.; Giglou, A. Effect of Trichoderma isolates on tomato seedling growth response and nutrient uptake. Afr. J. Biotechnol. 2011, 10, 5850–5855. [Google Scholar]
- Al-Ezerjawi, N.H.; Kadhim, J.H. Effect of two isolates of Trichoderma harzianum on total nitrogen, chlorophyll a and b contents and yield of wheat (Triticum aestivum. L.) class Eba’a-95. Int. J. Sci. Res. 2014, 3, 1078–1083. [Google Scholar]
- Badar, R.; Qureshi, S.A. Comparative effect of Trichoderma hamatum and host-specific Rhizobium species on growth of Vigna mungo. J. Appl. Pharm. Sci. 2012, 2, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Do Vale, L.H.F.; Filho, E.X.F.; Miller, R.N.G.; Ricart, C.A.O.; De Sousa, M.V. Cellulase systems in Trichoderma: An overview. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2014; pp. 229–244. [Google Scholar]
- Błaszczyk, L.; Siwulski, M.; Sobieralski, K.; Lisiecka, J.; Jędryczka, M. Trichoderma spp.—Application and prospects for use in organic farming and industry. J. Plant Prot. Res. 2014, 54, 309–317. [Google Scholar] [CrossRef]
- Nosrati, R.; Owlia, P.; Saderi, H.; Olamaee, M.; Rasooli, I.; Akhavian, T.A. Correlation between nitrogen fixation rate and alginate productivity of an indigenous Azotobacter vinelandii from Iran. Iran J. Microbiol. 2012, 4, 153–159. [Google Scholar]
- Daiz-Barrera, A.; Soto, E. Biotechnological uses of Azotobacter vinelandii: Current state, limits and prospects. Afr. J. Biotechnol. 2010, 9, 5240–5250. [Google Scholar]
- Rubio, E.J.; Montecchia, M.S.; Tosi, M.; Cassán, F.D.; Perticari, A.; Correa, O.S. Genotypic characterization of Azotobacteria isolated from Argentinean soils and plant-growth-promoting traits of selected strains with prospects for biofertilizer production. Sci. World J. 2013, 2013, 519603. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, A.; Piccolo, A. Polymerization of dissolved humic substances catalyzed by peroxidase. Effects of pH and humic composition. Org. Geochem. 2002, 33, 281–294. [Google Scholar] [CrossRef]
- Seipke, R.F. Strain-level diversity of secondary metabolism in Streptomyces albus. PLoS ONE 2015, 10, e0116457. [Google Scholar] [CrossRef] [Green Version]
- Zaburannyi, N.; Rabyk, M.; Ostash, B.; Fedorenko, V.; Luzhetskyy, A. Insights into naturally minimised Streptomyces albus J1074 genome. BMC Genomics 2014, 15, 97. [Google Scholar] [CrossRef] [Green Version]
- Nayaka, S.; Vidyasagar, G.M. Development of eco-friendly bio-fertilizer using feather compost. Ann. Plant Sci. 2013, 2, 238–244. [Google Scholar]
- Domenech, J.; Reddy, M.S.; Kloepper, J.W.; Ramos, B.; Gutierrez-Mañero, J. Combined application of the biological product LS213 with Bacillus, Pseudomonas or Chryseobacterium for growth promotion and biological control of soil-borne diseases in pepper and tomato. BioControl 2006, 51, 245–258. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Deaker, R.; Kennedy, I.R.; Roughley, R.J. The positive yield response of field-grown rice to inoculation with a multi-strain biofertiliser in the Hanoi area, Vietnam. Symbiosis 2003, 35, 231–245. [Google Scholar]
- Pierson, E.A.; Weller, D.M. Use of mixtures of fluorescent pseudomonads to suppress take-all and improve the growth of wheat. Phytopathology 1994, 84, 940–947. [Google Scholar] [CrossRef]
- Duffy, B.K.; Simon, A.; Weller, D.M. Combination of Trichoderma koningii with fluorescent pseudomonads for control of take-all on wheat. Phytopathology 1996, 86, 188–194. [Google Scholar] [CrossRef]
- Anith, K.N.; Manomohandas, T.P. Combined application of Trichoderma harzianum and Alcaligenes sp. strain AMB 8 for controlling nursery rot disease of black pepper. Indian Phytopathol. 2001, 54, 335–339. [Google Scholar]
- El-Katatny, H.M. Enzyme production and nitrogen fixation by free, immobilized and coimmobilized inoculants of Trichoderma harzianum and Azospirillum brasilense and their possible role in growth promotion of tomato. Food Technol. Biotechnol. 2010, 48, 161–174. [Google Scholar]
- Maketon, M.; Apisitsantikul, J.; Siriraweekul, C. Greenhouse evaluation of Bacillus subtilis AP-01 and Trichoderma harzianum AP-001 in controlling tobacco diseases. Braz. J. Microbiol. 2008, 39, 296–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabie, G.H.; Almadini, A.M. Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr. J. Biotechnol. 2005, 4, 210–222. [Google Scholar]
- Singh, S.; Kapoor, K.K. Inoculation with phosphate-solubilizing microorganisms and a vesicular-arbuscular mycorrhizal fungus improves dry matter yield and nutrient uptake by wheat grown in a sandy soil. Biol. Fertil. Soils 1999, 28, 139–144. [Google Scholar] [CrossRef]









| Plant Pathogens | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Examined Trichoderma Strains | FSSC SZMC 11057F | FSSC SZMC 6241J | FSSC SZMC 11067F | FSSC SZMC 11070F | Armillaria mellea MUCL 31056 | Armillaria ostoyae SZMC 23080 | Armillaria gallica SZMC 23076 | Alternaria alternata SZMC 16085 | Phoma cucurbitacearum SZMC 16088 | Botrytis cinerea SZMC 6244J | Rhizoctonia solani SZMC 6252J |
| T. asperellum SZMC 20866 | 79.77 ± 1.22 | 83.71 ± 1.35 | 71.38 ± 3.04 | 75.61 ± 5.35 | 33.91 ± 6.67 | 100.00 ± 0.00 | 100.00 ± 0.00 | 64.32 ± 0.53 | 81.36 ± 1.26 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| T. asperellum SZMC 20786 | 77.76 ± 0.96 | 83.50 ± 6.97 | 88.84 ± 5.48 | 85.76 ± 2.12 | 47.68 ± 5.22 | 92.33 ± 3.51 | 100.00 ± 0.00 | 65.77 ± 1.42 | 80.63 ± 0.50 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| T. atroviride SZMC 20780 | 62.41 ± 2.90 | 63.39 ± 5.10 | 37.63 ± 2.15 | 51.19 ± 0.96 | 93.76 ±2.89 | 100.00 ± 0.00 | 100.00 ± 0.00 | 51.43 ± 0.42 | 59.21 ± 1.37 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| T. atroviride SZMC 20781 | 63.91 ± 0.76 | 71.32 ± 4.40 | 39.93 ± 2.14 | 48.39 ± 2.53 | 97.43 ± 3.07 | 98.02 ± 2.00 | 100.00 ± 0.00 | 54.16 ± 0.27 | 56.82 ± 1.20 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| T. gamsii SZMC 20783 | 60.82 ± 1.42 | 67.42 ± 0.79 | 39.97 ± 1.74 | 59.36 ± 1.34 | 82.21 ± 9.56 | 100.00 ± 0.00 | 100.00 ± 0.00 | 49.22 ± 2.06 | 60.14 ± 0.75 | 50.70 ± 7.26 | 100.00 ± 0.00 |
| T. hamatum SZMC 20784 | 68.22 ± 0.73 | 64.10 ± 0.67 | 47.88 ± 1.75 | 62.52 ± 0.63 | 96.71 ± 3.41 | 97.00 ± 0.00 | 92.75 ± 2.95 | 61.28 ± 1.38 | 67.96 ± 1.19 | 46.82 ± 1.06 | 100.00 ± 0.00 |
| T. guizhouense SZMC 20761 | 63.02 ± 5.40 | 58.81 ± 1.72 | 63.39 ± 1.69 | 64.92 ± 0.87 | 96.70 ± 3.23 | 99.93 ± 0.11 | 100.00 ± 0.00 | 60.77 ± 1.06 | 61.19 ± 1.35 | 44.20 ± 4.21 | 100.00 ± 0.00 |
| T. atrobrunneum SZMC 20869 | 42.19 ± 2.53 | 47.46 ± 2.08 | 37.83 ± 0.17 | 38.40 ± 0.95 | 54.38 ± 1.98 | 100.00 ± 0.00 | 88.43 ± 4.27 | 18.02 ± 1.02 | 14.92 ± 1.89 | 42.26 ± 4.55 | 74.87 ± 7.34 |
| T. guizhouense SZMC 20762 | 63.69 ± 1.23 | 58.47 ± 5.30 | 47.91 ± 0.37 | 59.77 ± 1.79 | 97.42 ± 2.4 | 100.00 ± 0.00 | 100.00 ± 0.00 | 59.33 ± 0.53 | 57.84 ± 1.95 | 49.43 ± 3.03 | 100.00 ± 0.00 |
| T. virens SZMC 20779 | 40.66 ± 1.51 | 37.20 ± 1.38 | 53.43 ± 0.62 | 49.10 ± 2.91 | 53.48 ± 2.76 | 100.00 ± 0.00 | 86.19 ± 5.77 | 25.07 ± 2.33 | 35.59 ± 1.91 | 45.32 ± 8.37 | 62.37 ± 5.36 |
| Parameter | Measure | Sampling Time | Mean ± SD | |
|---|---|---|---|---|
| Control Rows | Treated Rows | |||
| Humus | m/m % | I. | 1.89 ± 0.04 | 1.98 ± 0.11 |
| W | II. | 1.57 ± 0.09 | 1.90 ± 0.33 | |
| III. | 1.90 ± 0.10 | 1.93 ± 0.19 | ||
| P2O5 | mg kg−1 | I. | 2478.33 ± 182.57 | 1895.88 ± 125.12 |
| II. | 1677.67 ± 69.04 | 1546.88 ± 135.31 | ||
| III. | 1694.00 ± 86.41 | 1145.25 ± 368.56 | ||
| K2O | mg kg−1 | I. | 702.67 ± 23.70 | 558.00 ± 76.90 * |
| II. | 701.67 ± 26.66 | 451.75 ± 64.14 *** | ||
| III. | 821.33 ± 54.63 | 480.13 ± 85.53 **** | ||
| SO4-S | mg kg−1 | I. | 16.33 ± 0.47 | 17.00 ± 1.22 |
| II. | 15.00 ± 0.82 | 16.88 ± 2.32 | ||
| III. | 7.33 ± 0.94 | 7.25 ± 2.11 | ||
| NOx-N | mg kg−1 | I. | 3.93 ± 0.54 | 4.68 ± 0.96 |
| II. | 4.67 ± 1.96 | 4.64 ± 1.25 | ||
| III. | 5.17 ± 0.66 | 5.24 ± 1.96 | ||
| Na | mg kg−1 | I. | 49.33 ± 0.94 | 45.00 ± 4.00 |
| II. | 45.00 ± 12.75 | 25.88 ± 12.69 * | ||
| III. | 46.33 ± 4.11 | 33.88 ± 5.37 | ||
| Mg | mg kg−1 | I. | 209.67 ± 2.62 | 220.75 ± 2.59 |
| II. | 194.33 ± 8.22 | 194.75 ± 12.16 | ||
| III. | 229.00 ± 11.22 | 213.38 ± 25.70 | ||
| Mn | mg kg−1 | I. | 14.33 ± 2.05 | 10.75 ± 0.66 |
| II. | 19.33 ± 1.25 | 17.63 ± 1.58 | ||
| III. | 19.67 ± 1.25 | 15.88 ± 3.18 * | ||
| Zn | mg kg-1 | I. | 6.20 ± 0.78 | 5.75 ± 0.72 |
| II. | 5.70 ± 0.37 | 6.44 ± 0.74 | ||
| III. | 4.73 ± 0.40 | 5.11 ± 1.16 | ||
| Cu | mg kg-1 | I. | 6.23 ± 1.11 | 4.36 ± 0.44 |
| II. | 8.20 ± 0.33 | 7.76 ± 0.44 | ||
| III. | 9.20 ± 1.55 | 9.15 ± 3.00 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allaga, H.; Bóka, B.; Poór, P.; Nagy, V.D.; Szűcs, A.; Stankovics, I.; Takó, M.; Manczinger, L.; Vágvölgyi, C.; Kredics, L.; et al. A Composite Bioinoculant Based on the Combined Application of Beneficial Bacteria and Fungi. Agronomy 2020, 10, 220. https://doi.org/10.3390/agronomy10020220
Allaga H, Bóka B, Poór P, Nagy VD, Szűcs A, Stankovics I, Takó M, Manczinger L, Vágvölgyi C, Kredics L, et al. A Composite Bioinoculant Based on the Combined Application of Beneficial Bacteria and Fungi. Agronomy. 2020; 10(2):220. https://doi.org/10.3390/agronomy10020220
Chicago/Turabian StyleAllaga, Henrietta, Bettina Bóka, Péter Poór, Viktor Dávid Nagy, Attila Szűcs, Istvánné Stankovics, Miklós Takó, László Manczinger, Csaba Vágvölgyi, László Kredics, and et al. 2020. "A Composite Bioinoculant Based on the Combined Application of Beneficial Bacteria and Fungi" Agronomy 10, no. 2: 220. https://doi.org/10.3390/agronomy10020220