Light and Temperature as Dual Stimuli Lead to Self-Assembly of Hyperbranched Azobenzene-Terminated Poly(N-isopropylacrylamide)
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Analysis and Characterization
2.3. Synthesis of 4-Propoxy-4′-Hydroxyazobenzene (C3H7O-Azo-OH), 4-Methoxy-4′-Hydroxyazobenzene (CH3O-Azo-OH), 4-Hydroxyazobenzene (Azo-OH), and 4-Carboxyl-4′-Hydroxyazobenzene (HOOC-Azo-OH)
2.4. Synthesis of 4-Propoxy-4′-(2-Bromopropionyloxy)azobenzene (C3H7O-Azo-Br), 4-Methoxy-4′-(2-Bromopropionyloxy)azobenzene (CH3O-Azo-Br), 4-(2-Bromopropionyloxy)azobenzene (Azo-Br), and 4-Carboxyl-4′-(2-Bromopropionyloxy)azobenzene (HOOC-Azo-Br)
2.5. Synthesis of Hyperbranched Poly(N-isopropylacrylamide)s (HBPNIPAM-Azo-OC3H7, HBPNIPAM-Azo-OCH3, HBPNIPAM-Azo, and HBPNIPAM-Azo-COOH) and Linear Poly(N-isopropylacrylamide) (LPNIPAM)
3. Results and Discussion
3.1. Preparation of Hyperbranched Poly(N-isopropylacrylamide)s End-Capped with Different Azobenzene Chromophores (HBPNIPAM-Azo-OC3H7, HBPNIPAM-Azo-OCH3, HBPNIPAM-Azo, and HBPNIPAM-Azo-COOH)
3.2. Photoisomerization Behavior of Hyperbranched Poly(N-isopropylacrylamide)s End-Capped with Different Azobenzene Chromophores (HBPNIPAM-Azo-OC3H7, HBPNIPAM-Azo-OCH3, HBPNIPAM-Azo, and HBPNIPAM-Azo-COOH)
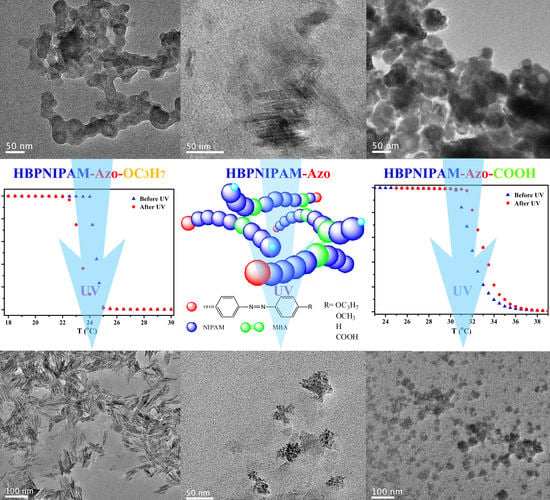
3.3. Self-Assembly of Hyperbranched Poly(N-isopropylacrylamide)s End-Capped with Different Azobenzene Chromophores (HBPNIPAM-Azo-OC3H7, HBPNIPAM-Azo-OCH3, HBPNIPAM-Azo, and HBPNIPAM-Azo-COOH)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Heskins, M.; Gillet, J.E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Wycisk, A.; Döring, A.; Schneider, M.; Schönhoff, M.; Kuckling, D. Synthesis of β-cyclodextrin-based star block copolymers with thermo-responsive behavior. Polymers 2015, 7, 921–938. [Google Scholar] [CrossRef]
- You, Y.Z.; Hong, C.Y.; Pan, C.Y.; Wang, P.H. Synthesis of a dendritic core-shell nanostructure with a temperature-sensitive shell. Adv. Mater. 2004, 16, 1953–1957. [Google Scholar] [CrossRef]
- Lutz, J.F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Kawano, T.; Niidome, Y.; Mori, T.; Katayama, Y.; Niidome, T. PNIPAM gel-coated gold nanorods for targeted delivery responding to a near-infrared laser. Bioconj. Chem. 2009, 20, 209–212. [Google Scholar] [CrossRef] [PubMed]
- You, Y.Z.; Kalebaila, K.K.; Brock, S.L.; Oupický, D. Temperature-controlled uptake and release in PNIPAM-modified porous silica nanoparticles. Chem. Mater. 2008, 20, 3354–3359. [Google Scholar] [CrossRef]
- Klaikherd, A.; Nagamani, C.; Thayumanavan, S. Multi-stimuli sensitive amphiphilic block copolymer assemblies. J. Am. Chem. Soc. 2009, 131, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.V.; Wesley, R.A.; Luginbuhl, R.; Denton, D.D.; Ratner, B.D. Plasma polymerized N-Isopropylacrylamide: Synthesis and characterization of a smart thermally responsive coating. Biomacromolecules 2001, 2, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.H.; Zuo, F.; Zheng, Z.H.; Cheng, X.; Ding, X.B.; Peng, Y.X. Tunable smart surface of gold nanoparticles achieved by light-controlled molecular recognition effection. Macromol. Rapid Commun. 2008, 18, 149–154. [Google Scholar] [CrossRef]
- Chen, T.; Fang, Q.; Zhong, Q.; Chen, Y.; Wang, J. Synthesis and thermosensitive behavior of polyacrylamide copolymers and their applications in smart textiles. Polymers 2015, 7, 909–920. [Google Scholar] [CrossRef]
- Bradley, C.; Jalili, N.; Nett, S.K.; Chu, L.Q.; Forch, R.; Gutmann, J.S.; Berger, R. Response characteristics of thermoresponsive polymers using nanomechanical cantilever sensors. Macromol. Chem. Phys. 2009, 210, 1339–1345. [Google Scholar] [CrossRef]
- Etika, K.C.; Jochum, F.D.; Cox, M.A.; Schattling, P.; Theato, P.; Grunlan, J.C. Nanotube friendly poly(N-isopropylacrylamide). Macromol. Rapid Commun. 2010, 31, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, Y.W.; Huang, W.; Zhou, Y.F.; Yan, D.Y. Terminal modification with 1-adamantylamine to endow hyperbranched polyamidoamine with thermo-/pH-responsive properties. Macromol. Rapid Commun. 2008, 29, 1746–1751. [Google Scholar] [CrossRef]
- Li, G.; Shi, L.; An, Y.; Zhang, W.; Ma, R. Double-responsive core-shell-corona micelles from self-assembly of diblock copolymer of poly(t-butyl acrylate-co-acrylic acid)-b-poly(N-isopropylacrylamide). Polymer 2006, 47, 4581–4587. [Google Scholar] [CrossRef]
- Zhang, W.D.; Zhang, W.; Cheng, Z.P.; Zhou, N.C.; Zhu, J.; Zhang, Z.B.; Chen, G.J.; Zhu, X.L. Synthesis and aggregation behaviors of nonlinear multiresponsive, multihydrophilic block copolymers. Macromolecules 2011, 44, 3366–3373. [Google Scholar] [CrossRef]
- Men, Y.J.; Drechsler, M.; Yuan, J.Y. Double-stimuli-responsive spherical polymer brushes with a poly(ionic liquid) core and a thermoresponsive shell. Macromol. Rapid Commun. 2013, 34, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Florian, D.J.; Theato, P. Temperature- and light-responsive polyacrylamides prepared by a double polymer analogous reaction of activated ester polymers. Macromolecules 2009, 42, 5941–5945. [Google Scholar]
- Li, Y.B.; He, Y.N.; Tong, X.L.; Wang, X.G. Photoinduced deformation of amphiphilic azo polymer colloidal spheres. J. Am. Chem. Soc. 2005, 127, 2402–2403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Light-responsive block copolymer micelles. Macromolecules 2012, 45, 3647–3657. [Google Scholar] [CrossRef]
- Kungwatchakun, D.; Irie, M. Photoresponsive polymers. Photocontrol of the phase separation temperature of aqueous solutions of poly-[N-isopropylacrylamide-co-N-(4-phenylazophenyl)acrylamide]. Makromol. Chem. Rapid Commun. 1988, 9, 243–246. [Google Scholar] [CrossRef]
- He, J.; Tremblay, L.; Lacelle, S.; Zhao, Y. How can photoisomerization of azobenzene induce a large cloud point temperature shift of PNIPAM? Polym. Chem. 2014, 5, 5403–5411. [Google Scholar] [CrossRef]
- Jochumab, F.D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Lin, L.; Yan, Z.; Yu, Y.L. Dual responsive block copolymer micelles functionalized by NIPAM and azobenzene. Macromol. Rapid Commum. 2010, 31, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Tamaoki, N. Synthesis and photoinduced phase transitions of poly(N-isopropylacrylamide) derivative functionalized with terminal azobenzene units. Macromolecules 2007, 40, 5129–5132. [Google Scholar] [CrossRef]
- Jochum, F.D.; Borg, L.Z.; Roth, P.J.; Theato, P. Thermo- and light-responsive polymers containing photoswitchable azobenzene end groups. Macromolecules 2009, 42, 7854–7862. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, X.S.; Wang, R.; Yin, J. Multistimuli responsive polymer nanoparticles on the basis of the amphiphilic azobenzene-contained hyperbranched poly(ether amine) (hPEA-AZO). Macromolecules 2010, 43, 10457–10465. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.J.; Yuan, Y.; Jiang, S.Z.; Yao, Y.F.; Chen, Y. Thermo-, pH-, and light-responsive supramolecular complexes based on a thermoresponsive hyperbranched polymer. ACS Macro. Lett. 2013, 2, 67–71. [Google Scholar] [CrossRef]
- Ciampolini, M.; Nardi, N. Five-coordinated high-spin complexes of bivalent cobalt, nickel, and copper with tris(2-dimethylaminoethyl)amine. Inorg. Chem. 1966, 5, 41–44. [Google Scholar] [CrossRef]
- Fréchet, J.M. J.; Gitsov, I.; Aoshima, S.; Leduc, M.R.; Grubbs, R.B. Self-condensing vinyl polymerization: An approach to dendritic materials. Science 1995, 269, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.; Chaplinski, V.; Braslau, R.; Hawker, C.J. Development of a universal alkoxyamine for “Living” free radical polymerizations. J. Am. Chem. Soc. 1999, 121, 3904–3920. [Google Scholar] [CrossRef]
- Zou, P.; Yang, L.P.; Pan, C.Y. One-pot synthesis of linear-hyperbranched diblock copolymers via self-condensing vinyl polymerization and ring opening polymerization. J. Polym. Sci. A Polym. Chem. 2008, 46, 7628–7636. [Google Scholar] [CrossRef]
- Li, Y.; Armes, S.P. Synthesis and chemical degradation of branched vinyl polymers prepared via ATRP: Use of a cleavable disulfide-based branching agent. Macromolecules 2005, 38, 8155–8162. [Google Scholar] [CrossRef]
- Zhang, C.B.; Zhou, Y.; Liu, Q.; Li, S.X.; Perrier, S.; Zhao, Y.L. Facile synthesis of hyperbranched and star-shaped polymers by RAFT polymerization based on a polymerizable trithiocarbonate. Macromolecules 2011, 44, 2034–2049. [Google Scholar] [CrossRef]
- Li, F.; Xue, X.Q.; Huang, W.Y.; Yang, H.J.; Jiang, B.B.; Zheng, Y.L.; Zhang, D.L.; Fang, J.B.; Chen, J.H.; Kong, L.Z. Ultrafast preparation of branched poly(methyl acrylate) through single electron transfer living radical polymerization at room temperature. Polym. Eng. Sci. 2014, 7, 1579–1584. [Google Scholar] [CrossRef]
- Yang, H.J.; Bai, T.; Xue, X.Q.; Huang, W.Y.; Chen, J.H.; Qian, X.L.; Zhang, G.Z.; Jiang, B.B. A simple route to vinyl-functionalized hyperbranched polymers: Self-condensing anionic copolymerization of allyl methacrylate and hydroxyethyl methacrylate. Polymer 2015, 72, 63–68. [Google Scholar] [CrossRef]
- Xue, X.Q.; Yang, J.; Huang, W.Y.; Yang, H.J.; Jiang, B.B. Synthesis of hyperbranched poly(ε-caprolactone) containing terminal azobenzene structure via combined ring-opening polymerization and “click” chemistry. Polymers 2015, 7, 1248–1268. [Google Scholar] [CrossRef]
- Xue, X.Q.; Wang, Y.L.; Huang, W.Y.; Yang, H.J.; Chen, J.H.; Fang, J.B.; Yang, Y.; Kong, L.Z.; Jiang, B.B. New insight into the ATRP of monovinyl and divinyl monomers. Macromol. Chem. Phys. 2015, 216, 1555–1561. [Google Scholar] [CrossRef]
- Yang, H.J.; Bai, T.; Xue, X.Q.; Huang, W.Y.; Chen, J.H.; Qian, X.L.; Zhang, G.Z.; Jiang, B.B. A versatile strategy for synthesis of hyperbranched polymers with commercially available methacrylate inimer. RSC Adv. 2015, 5, 60401–60408. [Google Scholar] [CrossRef]
- Jiang, Q.M.; Huang, W.Y.; Yang, H.J.; Xue, X.Q.; Jiang, B.B.; Zhang, D.L.; Fang, J.B.; Chen, J.H.; Yang, Y. Radical emulsion polymerization with chain transfer monomer: An approach to branched vinyl polymers with high molecular weight and relatively narrow polydispersity. Polym. Chem. 2014, 5, 1863–1873. [Google Scholar] [CrossRef]
- Xue, X.Q.; Li, F.; Huang, W.Y.; Yang, H.J.; Jiang, B.B.; Zheng, Y.L.; Zhang, D.L.; Fang, J.B.; Kong, L.Z.; Zhai, G.Q.; et al. Quadrangular prism: A unique self-assembly from amphiphilic hyperbranched PMA-b-PAA. Macromol. Rapid. Commun. 2014, 35, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Gao, H.F. New method to access hyperbranched polymers with uniform structure via one-pot polymerization of inimer in microemulsion. J. Am. Chem. Soc. 2012, 134, 15680–15683. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.D.; Huang, W.Y.; Zhang, D.L.; Gong, F.H.; Liu, C.L.; Yang, Y.; Chen, J.H.; Jiang, B.B. Studies on the development of branching in ATRP of styrene and acrylonitrile in the presence of divinylbenzene. Polymer 2008, 49, 4101–4108. [Google Scholar]
- Huang, W.Y.; Zheng, Y.L.; Jiang, B.B.; Zhang, D.L.; Chen, J.H.; Yang, Y.; Liu, C.L.; Zhai, G.Q.; Kong, L.Z.; Gong, F.H. Studies on the atom transfer radical branching copolymerization of styrene and acrylonitrile with divinyl benzene as the branching agent. Macromol. Chem. Phys. 2010, 211, 2211–2217. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, W.Y.; Xue, X.Q.; Yang, H.J.; Jiang, B.B.; Zhang, D.L.; Fang, J.B.; Chen, J.H.; Yang, Y.; Zhai, G.Q.; et al. Synthesis of hyperbranched and highly branched methacrylates by self-condensing group transfer copolymerization. Macromolecules 2012, 45, 4092–4100. [Google Scholar] [CrossRef]
- Huang, W.Y.; Liu, C.; Yang, H.J.; Xue, X.Q.; Jiang, B.B.; Zhang, D.L.; Kong, L.Z.; Zhang, Y.; Komarneni, S. Facile synthesis of highly branched poly(acrylonitrile-co-vinyl acetate)s with low viscosity and high thermal stability via radical aqueous solution polymerization. Polym. Chem. 2014, 5, 3326–3334. [Google Scholar] [CrossRef]
- Xia, Y.; Yin, X.C.; Burke, N.A.D.; Stö1ver, H.D.H. Thermal response of narrow-disperse poly(N-isopropylacrylamide) prepared by atom transfer radical polymerization. Macromolecules 2005, 38, 5937–5943. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Shipp, D.A.; Wang, J.L.; Grimaud, T.; Patten, T.E. Utilizing halide exchange to improve control of atom transfer radical polymerization. Macromolecules 1998, 31, 6836–6840. [Google Scholar] [CrossRef]
- Xue, X.Q.; Yang, J.; Huang, W.Y.; Yang, H.J.; Jiang, B.B.; Li, F.; Jiang, Y. Dual thermo- and light-responsive nanorods from self-assembly of the 4-propoxyazobenzene-terminated poly(N-isopropylacrylamide) in aqueous solution. Polymer 2015, 73, 195–204. [Google Scholar] [CrossRef]
- Huang, W.Y.; Yang, H.J.; Xue, X.Q.; Jiang, B.B.; Chen, J.H.; Yang, Y.; Pu, H.T.; Liu, Y.; Zhang, D.L.; Kong, L.Z.; et al. Polymerization behaviors and polymer branching structures in ATRP of monovinyl and divinyl monomers. Polym. Chem. 2013, 4, 3204–3211. [Google Scholar] [CrossRef]
- Xue, X.Q.; Zhu, J.; Zhang, Z.B.; Zhou, N.C.; Tu, Y.F.; Zhu, X.L. Soluble main-chain azobenzene polymers via thermal 1,3-dipolar cycloaddition: Preparation and photoresponsive behavior. Macromolecules 2010, 43, 2704–2712. [Google Scholar] [CrossRef]
- Xue, X.Q.; Zhu, J.; Zhang, Z.B.; Cheng, Z.P.; Tu, Y.F.; Zhu, X.L. Synthesis and characterization of azobenzene-functionalized poly(styrene)-b-poly(vinyl acetate) via the combination of RAFT and “click” Chemistry. Polymer 2010, 51, 3083–3090. [Google Scholar] [CrossRef]
- Xue, X.Q.; Yang, J.; Huang, W.Y.; Yang, H.J.; Jiang, B.B. Preparation and characterization of novel side-chain azobenzene polymers containing tetrazole group. React. Funct. Polym. 2015, 96, 61–70. [Google Scholar] [CrossRef]
- Huang, T.C.; Chen, Y.Y.; Chu, C.C.; Hsiao, V.K.S. Optothermal switching of cholesteric liquid crystals: A Study of azobenzene derivatives and laser wavelengths. Materials 2015, 8, 6071–6084. [Google Scholar] [CrossRef]
- Caruso, U.; Diana, R.; Fort, A.; Panunzi, B.; Roviello, A. Synthesis of polymers containing second order NLO-active thiophene and thiazole based chromophores. Macromol. Symp. 2006, 234, 87–93. [Google Scholar] [CrossRef]









| Sample | Time c (h) | Conv.d (%) | Mn GPC e (g·mol−1) | Mw/Mn e | Mw MALLS f (g mol−1) | α g | g′ h | LCST i °C | ΔT j |
|---|---|---|---|---|---|---|---|---|---|
| LPNIPAM a | 60 | 94.3 | 4100 | 1.23 | - | 0.90 | 1.00 | 32.0 | 0 |
| HBPNIPAM-Azo-OC3H7 b | 24 | 98.8 | 7300 | 2.13 | 65,200 | 0.26 | 0.60 | 24.0 | −2.0 |
| HBPNIPAM-Azo-OCH3 b | 45 | 97.0 | 8400 | 1.82 | 50,400 | 0.28 | 0.73 | 25.5 | −1.0 |
| HBPNIPAM-Azo b | 48 | 98.1 | 8800 | 1.78 | 57,900 | 0.23 | 0.65 | 27.0 | 0 |
| HBPNIPAM-Azo-COOH b | 23 | 92.0 | 7400 | 2.05 | 52,500 | 0.25 | 0.59 | 30.5 | 1.0 |
| Sample | λmax (trans) a nm | λmax (cis) b nm | ke c × 102 s−1 | kH d × 103 s−1 | [trans] e % |
|---|---|---|---|---|---|
| HBPNIPAM-Azo-OC3H7 | 344 | 431 | 17.40 | 8.96 | 38.4 |
| HBPNIPAM-Azo-OCH3 | 350 | 427 | 23.78 | 4.96 | 36.5 |
| HBPNIPAM-Azo | 322 | 425 | 2.28 | 3.52 | 33.6 |
| HBPNIPAM-Azo-COOH | 329 | 425 | 4.50 | 4.69 | 40.0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Yang, J.; Xia, Y.; Wang, X.; Xue, X.; Yang, H.; Wang, G.; Jiang, B.; Li, F.; Komarneni, S. Light and Temperature as Dual Stimuli Lead to Self-Assembly of Hyperbranched Azobenzene-Terminated Poly(N-isopropylacrylamide). Polymers 2016, 8, 183. https://doi.org/10.3390/polym8050183
Huang W, Yang J, Xia Y, Wang X, Xue X, Yang H, Wang G, Jiang B, Li F, Komarneni S. Light and Temperature as Dual Stimuli Lead to Self-Assembly of Hyperbranched Azobenzene-Terminated Poly(N-isopropylacrylamide). Polymers. 2016; 8(5):183. https://doi.org/10.3390/polym8050183
Chicago/Turabian StyleHuang, Wenyan, Jing Yang, Yunqing Xia, Xuezi Wang, Xiaoqiang Xue, Hongjun Yang, Guifang Wang, Bibiao Jiang, Fang Li, and Sridhar Komarneni. 2016. "Light and Temperature as Dual Stimuli Lead to Self-Assembly of Hyperbranched Azobenzene-Terminated Poly(N-isopropylacrylamide)" Polymers 8, no. 5: 183. https://doi.org/10.3390/polym8050183