Acid Ionic Liquids as a New Hardener in Urea-Glyoxal Adhesive Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of UG Resin
2.2. [HNMP][HSO4] Synthesis
2.3. Addition of Catalysts to UG Resin
2.4. Measurements of the pH and Gel Time
2.5. DSC Analysis
2.6. CP-MAS 13C NMR Analysis
2.7. MALDI TOF-MS Analysis
2.8. Particleboard Manufacturing
2.9. Panel Testing
2.10. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Effect of the [HNMP] HSO4 Content on the pH and Gel Time of the UG Resin
3.2. DSC Results
3.3. 13C NMR of UG Resin


3.4. MALDI TOF Results







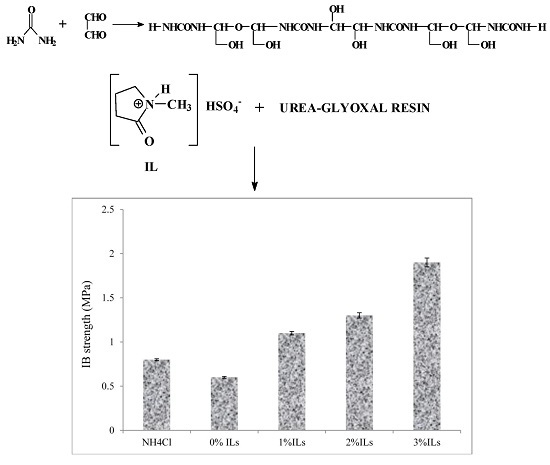
3.5. Properties of Particleboards
3.6. Morphology Characteristics
4. Conclusions
- Acidic ionic liquids, such as a halogen-free and ecofriendly ionic liquid, is an effective catalyst for urea-glyoxal resins.
- SEM micrographs showed that the wood-based panels containing an acidic ionic liquid had a smooth and monotonous matrix.
- The particleboard panels prepared with an ionic liquid hardener had higher mechanical strength and better dimensional stability compared to those made from UG resin containing NH4Cl.
- The gel time of the UG resin was accelerated with increasing [HNMP] HSO4 content.
- MALDI TOF mass spectroscopy indicated that UG resins need to overcome a high energy of activation on curing.
- DSC analysis showed that the addition of IL to the UG resin decrease the energy of activation of the curing reaction to render possible cross-linking.
Author Contributions
Conflicts of Interest
References
- Deng, S.; Du, G.; Li, X.; Zhang, J.; Pizzi, A. Performance and reaction mechanism of zero formaldehyde-emission urea-glyoxal (UG) resin. J. Taiwan Inst. Chem. Eng. 2014, 45, 2029–2038. [Google Scholar] [CrossRef]
- Deng, S.; Pizzi, A.; Du, G.; Zhang, J.; Zhang, J. Synthesis, structure, and characterization of Glyoxal-Urea-Formaldehyde cocondensed resins. J. Appl. Polym. Sci. 2014, 131, 41009–41019. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, S.Y.; Deng, J.; Wang, S. Urea-formaldehyde-resin gel time as affected by the pH value, solid content, and catalyst. J. Appl. Polym. Sci. 2007, 103, 1566–1569. [Google Scholar] [CrossRef]
- Sun, Q.N.; Hse, C.Y.; Shupe, C.F. Effect of different catalysts on urea-formaldehyde resin synthesis. J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Deshmukh, K.M.; Qureshi, Z.S.; Patil, Y.P.; Bhanage, B.M. Ionic liquid [NMP]+HSO4: An efficient and recyclable catalyst for the synthesis of 1-Amidoalkyl-2-naphthols and 1-carbamatoalkyl-2-naphthols under solvent free conditions. Synthetic Commun. 2012, 42, 93–101. [Google Scholar] [CrossRef]
- Wang, X.; Han, M.; Wan, H.; Yang, C.; Guan, G. Study on extraction of thiophene from model gasoline with Brønsted acidic ionic liquids. Fron. Chem. Sci. Eng. 2011, 5, 107–112. [Google Scholar] [CrossRef]
- Pizzi, A.; Vosloo, R.; Cameron, A.; Orovan, E. Self- neutralizing acid-set PF wood adhesives. Holz. Roh. Werkst. 1986, 44, 229–234. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Kazemi Najafi, S.; Behrooz, R.; Pizzi, A. Improving urea formaldehyde resin properties by glyoxalated soda bagasse lignin. Holz. Roh. Werkst. 2015, 73, 77–85. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Pizzi, A.; Niyatzade, G. Reduction of formaldehyde emission from particleboard by phenolated kraft lignin. J. Adhes. 2015. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Kazemi-Najafi, S.; Behrooz, R. Influence of nanoclay on urea-glyoxalated lignin-formaldehyde resins for wood adhesive. J. Adhes. 2015. [Google Scholar] [CrossRef]
- European Committee for Standardization. European Norm EN 319: 1993 Perpendicular Tensile Strength on Particleboards and Fibreboards; European Committee for Standardization: Brussels, Belgium, 1993. [Google Scholar]
- European Committee for Standardization. European Norm EN 310: 1993 Wood-Based Panels—Determination of Modulus of Elasticity in Bending and of Bending Strength; European Committee for Standardization: Brussels, Belgium, 1993. [Google Scholar]
- ASTM. ASTM D 4442: 2007 Standard Test Methods for Direct Moisture Content Measurement of Wood and Wood-Base Materials; ASTM: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Mao, A.; Hassan, E.B.; Kim, M.G. Investigation of low mole ratio UF and UMF resins aimed at lowering the formaldehyde emission potential of wood composite boards. Bioresources 2013, 8, 2453–2469. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, S.Y.; Deng, J. Effect of wood acidity and catalyst on UF resin gel time. Holzforschung 2005, 58, 408–412. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, X.M.; Wan, H.; Liu, Y. Curing characteristics of urea–Formaldehyde resin in the presence of various amounts of wood extracts and catalysts. J. Appl. Polym. Sci. 2008, 107, 1555–1562. [Google Scholar] [CrossRef]
- Paiva, N.T.; Henriques, A.; Cruz, P.; Ferra, J.M.; Carvalho, L.H.; Magalhaes, F.D. Production of melamine fortified urea-formaldehyde resins with low formaldehyde emission. J. Appl. Polym. Sci. 2012, 124, 2311–2317. [Google Scholar] [CrossRef]
- Kardos, J.L.; Dudu, K.; Dave, R. Void growth and resin transport during processing of thermosetting—Matrix composites. Adv. Polym. Sci. 1986, 80, 101–123. [Google Scholar]
- Hse, C.Y.; Xia, Z.Y.; Tomita, B. Effects of reaction pH on properties and performance of urea-formaldehyde resins. Holzforchung 1994, 48, 527–532. [Google Scholar] [CrossRef]
- Taghyari, H.R.; Rangavar, H.; Bibalan, O.F. Effect of nanosilver on reduction of hot pressing time and improvement in physical and mechanical properties of particleboard. Bioresources 2011, 6, 4067–4075. [Google Scholar]












© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younesi-Kordkheili, H.; Pizzi, A. Acid Ionic Liquids as a New Hardener in Urea-Glyoxal Adhesive Resins. Polymers 2016, 8, 57. https://doi.org/10.3390/polym8030057
Younesi-Kordkheili H, Pizzi A. Acid Ionic Liquids as a New Hardener in Urea-Glyoxal Adhesive Resins. Polymers. 2016; 8(3):57. https://doi.org/10.3390/polym8030057
Chicago/Turabian StyleYounesi-Kordkheili, Hamed, and Antonio Pizzi. 2016. "Acid Ionic Liquids as a New Hardener in Urea-Glyoxal Adhesive Resins" Polymers 8, no. 3: 57. https://doi.org/10.3390/polym8030057