Functionalized Cellulose Networks for Efficient Oil Removal from Oil–Water Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
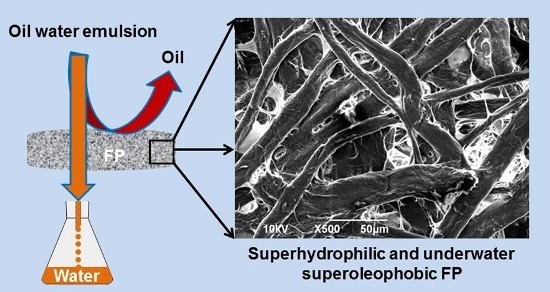
2.2. Fabrication of Superhydrophilic and Underwater Superoleophobic FP
2.3. Characterization Studies
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Porosity Measurements
2.3.3. Fourier Transform Infrared Spectroscopy-Attenuated Total Reflection (FTIR-ATR)
2.3.4. Mechanical Characterization
2.3.5. Wettability and Contact Angle Measurements
2.3.6. Separation of Oil from Oil-in-Water Non-Stabilized Emulsions
2.3.7. UV-vis Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wen, Q.; Di, J.; Jiang, L.; Yu, J.; Xu, R. Zeolite-coated mesh film for efficient oil–water separation. Chem. Sci. 2013, 4, 591–595. [Google Scholar] [CrossRef]
- Tao, S.; Wang, Y. Synthesis of hierarchically porous silica film with controllable surface wettability. Int. Nano Lett. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Calcagnile, P.; Fragouli, D.; Bayer, I.S.; Anyfantis, G.C.; Martiradonna, L.; Cozzoli, P.D.; Cingolani, R.; Athanassiou, A. Magnetically driven floating foams for the removal of oil contaminants from water. ACS Nano 2012, 6, 5413–5419. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/water separation with selective superantiwetting/superwetting surface materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zu, G.; Wang, X.; Yang, J.; Hu, J. Fabrication of oil–water separation filter paper by simple impregnation with fluorinated poly-acrylate emulsion. BioResources 2014, 9, 4421–4429. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Lu, Q. Filter paper with selective absorption and separation of liquids that differ in surface tension. ACS Appl. Mater. Interfaces 2010, 2, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.; Wang, S.; Shi, Y.; Li, J. Fabrication of coral-like superhydrophobic coating on filter paper for water–oil separation. Appl. Surf. Sci. 2012, 261, 764–769. [Google Scholar] [CrossRef]
- Rohrbach, K.; Li, Y.; Zhu, H.; Liu, Z.; Dai, J.; Andreasen, J.; Hu, L. A cellulose based hydrophilic, oleophobic hydrated filter for water/oil separation. Chem. Commun. 2014, 50, 13296–13299. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wang, Q.; Xiong, S.; Wang, Y. Turning low-cost filter papers to highly efficient membranes for oil/water separation by atomic-layer-deposition-enabled hydrophobization. Ind. Eng. Chem. Res. 2014, 53, 16516–16522. [Google Scholar] [CrossRef]
- Du, C.; Wang, J.; Chen, Z.; Chen, D. Durable superhydrophobic and superoleophilic filter paper for oil–water separation prepared by a colloidal deposition method. Appl. Surface Sci. 2014, 313, 304–310. [Google Scholar] [CrossRef]
- Feng, X.; Shi, Y.; Liu, J.; Yang, W. Fabrication of filter paper with tunable wettability and its application in oil–water separation. J. Sol-Gel Sci. Technol. 2015, 4, 3759–3767. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.H.; Ji, P.T.; Zhang, P.; Lia, Y.R.; Ji, S.T. Fabrication of superhydrophobic and superoleophilic textiles for oil–water separation. Appl. Surface Sci. 2013, 284, 464–471. [Google Scholar] [CrossRef]
- Liu, X.; Ge, L.; Li, W.; Wang, X.; Li, F. Layered double hydroxide functionalized textile for effective oil/water separation and selective oil adsorption. ACS Appl. Mater. Interfaces 2015, 7, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Seddighi, M.; Hejazi, S.M. Water–oil separation performance of technical textiles used for marine pollution disasters. Marine Pollut. Bull. 2015, 96, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, B.W.; Shi, Z.; Wang, D.; Jin, J.; Jiang, L. Nanowire-haired inorganic membranes with superhydrophilicity and underwater ultralow adhesive superoleophobicity for high-efficiency oil/water separation. Adv. Mater. 2013, 25, 4192–4198. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S.; Fragouli, D.; Attanasio, A.; Sorce, B.; Bertoni, G.; Brescia, R.; Corato, R.D.; Pellegrino, T.; Kalyva, M.; Sabella, S.; et al. Water-repellent cellulose fiber networks with multifunctional properties. ACS Appl. Mater. Interfaces 2011, 3, 4024–4031. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, F.; Bayer, I.S.; Fragouli, D.; Liakos, I.; Cingolani, R.; Athanassiou, A. Mechanical reinforcement and water repellency induced to cellulose sheets by a polymer treatment. Cellulose 2013, 20, 1501–1509. [Google Scholar] [CrossRef]
- Jang, I.; Song, S. Facile and precise flow control for a paper-based microfluidic device through varying paper permeability. Lab Chip 2015, 15, 3405–3412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; van Den Berg, A. Focus Lab on paper. Lab Chip 2008, 8, 1988–1991. [Google Scholar] [PubMed]
- Liu, Q.; Patel, A.A.; Liu, L. Superhydrophilic and underwater superoleophobic poly(sulfobetaine methacrylate)-grafted glass fiber filters for oil−water separation. ACS Appl. Mater. Interfaces 2014, 6, 8996–9003. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Fu, Q.; Wang, X.; Zhu, J.; Yu, J.; Sun, G.; Ding, B. Superelastic and superhydrophobic nanofiber-assembled cellular aerogels for effective separation of oil/water emulsions. ACS Nano 2015, 9, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.E.; Lalia, B.S.; Hilal, N.; Hashaikeh, R. Underwater superoleophobic cellulose/electrospun PVDF–HFP membranes for efficient oil/water separation. Desalination 2014, 344, 48–54. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Membr. Sci. 2008, 325, 427–437. [Google Scholar] [CrossRef]
- Spence, K.L.; Richard, A.; Venditti, R.A.; Rojas, O.J.; Pawlak, J.J.; Hubbe, M.A. Water vapor barrier properties of coated and filled microfibrillated cellulose composite films. BioResources 2011, 6, 4370–4388. [Google Scholar]
- Kumar, S.; Kumar, A. Study on petroleum derived waxes and their uses. Int. J. Res. Sci. Technol. 2013, 2, 1–8. [Google Scholar]
- Khwaldia, K. Water vapor barrier and mechanical properties of paper-sodium caseinate and paper-sodium caseinate-paraffin wax films. J. Food Biochem. 2010, 34, 998–1013. [Google Scholar] [CrossRef]
- Donhowe, G.; Fennema, O.R. Water vapor and oxygen permeability of wax films. J. Am. Oil Chem. Soc. 1993, 70, 867–873. [Google Scholar] [CrossRef]
- Rheingans, O.; Hugenberg, N.; Harris, J.R.; Fischer, K.; Maskos, M. Nanoparticles built of cross-linked heterotelechelic, amphiphilic poly(dimethylsiloxane)-b-poly(ethylene oxide) diblock copolymers. Macromolecules 2000, 33, 4780–4790. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Tu, M. Synthesis of porous methylphenylsiloxane/poly(dimethylsiloxane) composite using poly(dimethylsiloxane)–poly(ethylene oxide) (PDMS–PEO) as template. J. Mater. Sci. 2012, 47, 3350–3353. [Google Scholar] [CrossRef]
- Galin, M.; Mathis, A. Structural and thermodynamic study of dimethylsiloxane-ethylene oxide PDMS-PEO-PDMS triblock copolymers. Macromolecules 1981, 14, 677–683. [Google Scholar] [CrossRef]
- Luzinov, I.; Minko, S.; Tsukruk, V.V. Adaptive and responsive surfaces through controlled reorganization of interfacial polymer layers. Prog. Polym. Sci. 2004, 29, 635–698. [Google Scholar] [CrossRef]
- Yao, M.; Fang, J. Hydrophilic PEO-PDMS for microfluidic applications. Micromech. Microeng. 2012, 22, 1–6. [Google Scholar] [CrossRef]
- Chavan, A.A.; Li, H.; Scarpellini, A.; Marras, S.; Manna, L.; Athanassiou, A.; Fragouli, D. Elastomeric nanocomposite foams for the removal of heavy metal ions from water. ACS Appl. Mater. Interfaces 2015, 7, 14778–14784. [Google Scholar] [CrossRef] [PubMed]
- Adela, A.M.; El-Wahab, Z.H.A.; Ibrahim, A.A.; Al-Shemya, M.T. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part II: Physicochemical properties. Carbohydr. Polym. 2011, 83, 676–687. [Google Scholar] [CrossRef]
- Cunha, A.G.; Freire, C.; Silvestre, A.; Neto, C.P.; Gandini, A.; Belgacem, M.N.; Chaussy, D.; Beneventi, D. Preparation of highly hydrophobic and lipophobic cellulose fibers by a straightforward gas–solid reaction. J. Colloid Interface Sci. 2010, 344, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Gomez, N.A.; Abonia, R.; Hector Cadavid, H.; Vargas, I.H. Chemical and spectroscopic characterization of a vegetable oil used as dielectric coolant in distribution transformers. J. Braz. Chem. Soc. 2011, 22, 2292–2303. [Google Scholar] [CrossRef]
- ASTM D 2269. Standard Test Method for Evaluation of White Mineral Oils by Ultraviolet Absorption; ASTM International: West Conshohocken, PA, USA, 1988. [Google Scholar]








| Sample | SCA of water (°) air | SCA of DCM (°) air | SCA of DCM (°) underwater |
|---|---|---|---|
| Untreated | absorbed | absorbed | 134.7 ± 3.2 |
| PFW | 125.8 ± 2.3 | absorbed | 140.0 ± 1.6 |
| PDMS-b-PEO | absorbed | absorbed | 142.4 ± 0.6 |
| PFW/PDMS-b-PEO | absorbed | absorbed | 148.7 ± 6.4 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, U.C.; Fragouli, D.; Bayer, I.S.; Athanassiou, A. Functionalized Cellulose Networks for Efficient Oil Removal from Oil–Water Emulsions. Polymers 2016, 8, 52. https://doi.org/10.3390/polym8020052
Paul UC, Fragouli D, Bayer IS, Athanassiou A. Functionalized Cellulose Networks for Efficient Oil Removal from Oil–Water Emulsions. Polymers. 2016; 8(2):52. https://doi.org/10.3390/polym8020052
Chicago/Turabian StylePaul, Uttam C., Despina Fragouli, Ilker S. Bayer, and Athanassia Athanassiou. 2016. "Functionalized Cellulose Networks for Efficient Oil Removal from Oil–Water Emulsions" Polymers 8, no. 2: 52. https://doi.org/10.3390/polym8020052