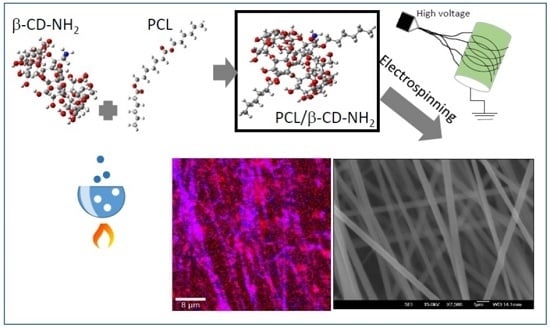
Polycaprolactone/Amino-β-Cyclodextrin Inclusion Complex Prepared by an Electrospinning Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 6-Monodeoxy-6-monoamino-β-cyclodextrin (β-CD-NH2)
2.2. Preparation of Inclusion Complex of PCL with β-CD and 6-Monodeoxy-6-monoamino-β-cyclodextrin (β-CD-NH2)
2.3. Theoretical Study
2.4. Electrospinning
3. Results
3.1. Synthesis of 6-Deoxy-6-amine-β-cyclodextrin
3.2. Inclusion Complexes β-CD and β-CD-NH2 with PCL and Electrospinning
3.3. Theoretical Study
3.4. Electrospinning
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of Polymeric Nanofibers forTissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.; Yarin, A.L.; Zussman, E.; Xu, H. Electrospinning of Nanofibers from Polymer Solutions and Melts. Adv. Appl. Mech. 2007, 41, 43–195. [Google Scholar]
- Gabriel, M.; Nazmi, K.; Dahm, M.; Zentner, A.; Vahl, C.-F.; Strand, D. Covalent RGD Modification of the Inner Pore Surface of Policaprolactone Scaffolds. J. Biomater. Sci. 2012, 23, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Hakeam, G.A.; Eid, K.; Sharaf, M.A.; Badr, Y.A.; Abbass, M.M.; Solouma, N.H. Laser Surface Modification of poly(ε-caprolactone) Scaffold for Artificial Skin Applications. Am. J. Biomed. Sci. 2013, 5, 1–13. [Google Scholar] [CrossRef]
- Sousa, I.; Mendes, A.; Pereira, R.F.; Bártolo, P.J. Collagen Surface Modified Poly(ε-caprolactone) Scaffolds With Improved Hydrophilicity and Cell Adhesion Properties. Mater. Lett. 2014, 134, 263–267. [Google Scholar] [CrossRef]
- Ji, Y.; Liang, K.; Shen, X.; Bowlin, G.L. Electrospinning and characterization of chitin nanofibril/polycaprolactone nanocomposite fiber mats. Carbohydr. Polym. 2014, 101, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, E.A.; de la Rosa, L.A.; Rodrigo-García, J.; Escobedo-González, R.; Mercado-Mercado, G.; Moyers-Montoya, E.; Vázquez-Flores, A.; Aguilar, G.A.G. Dual effect of β-cyclodextrin on the inhibition of apple polyphenol oxidase by 4-hexylresorcinol (HR) and methyl Jasmonate (MJ). Food Chem. 2007, 101, 1346–1356. [Google Scholar]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin–β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Carber, P.; Alvarez-Parrilla, E.; Meijide, F.; Seijas, J.A.; Rodríguez-Nunez, E.; Vázquez-Tato, J. Complexation of Sodium Cholate and Sodium Deoxycholate by β-Cyclodextrin and Derivatives. Langmuir 1999, 15, 5489–5495. [Google Scholar] [CrossRef]
- Li, J.; Loh, X.L. Cyclodextrin-based supramolecular architectures: Syntheses, structures, and applications for drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Saldias, C.; Gargallo, L.; Sandoval, C.; Leiva, A.; Radic, D.; Caballero, J.; Saavedra, M.; Gonzalez-Nilo, F.D. Inclusion complexes containing poly(ε-caprolactone)diol and cyclodextrins. Experimental and theorical studies. Polymer 2009, 50, 2926–2932. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Nishiyama, T.; Okada, M.; Kamachi, M.; Harada, A. Complex Formation of Poly(ε-caprolactone) with Cyclodextrins. Macromolecules 2000, 33, 4472–4477. [Google Scholar] [CrossRef]
- Brichkin, S.B.; Chernykh, E.V. Supramolecular Complexes Based on Cyclodextrins. High Energy Chem. 2010, 44, 83–100. [Google Scholar]
- Zhan, J.; Singh, A.; Zhang, Z.; Huang, L.; Elisseeff, J.H. Multifunctional aliphatic polyester nanofibers for tissue engineering. Biomatter 2012, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Gupta, B.S.; Tonelli, A.E. Poly(ε-caprolactone) Nanowebs Functionalized with α-and γ-Cyclodextrins. Biomacromolecules 2014, 15, 4122–4133. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Ormond, B.R.; Gupta, B.S.; Tonelli, A.E. Efficient wound odor removal by β-cyclodextrin functionalized poly(ε-caprolactone). J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Narayanan, G.; Gupta, B.S.; Tonelli, A.E. Enhanced mechanical properties of poly(ε-caprolactone) nanofibers produced by the addition of non-stoichiometric inclusion complexes of poly(ε-caprolactone) and α-cyclodextrin. Polymer 2015, 76, 321–330. [Google Scholar] [CrossRef]
- Narayanan, G.; Aguda, R.; Hartman, M.; Chung, C.C.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Fabrication and characterization of poly(ε-caprolactone)/α-cyclodextrin pseudorotaxane nanofibers. Biomacromolecules 2015, 17, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Byun, H.-S.; Bittrnan, R. An Improved Synthesis of 6-O-Monotosyi-6-deoxy-β-cyclodextrin. Tetrahedron Lett. 1998, 39, 2919–2920. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Liu, L.; Li, X.S.; Guo, Q.X.; Liu, Y.C. Hartree-Fock and Density Functional Theory Studies on the Molecular Recognition of the Cyclodextrin. Chin. Chem. Lett. 1999, 10, 1053–1056. [Google Scholar]
- Escobedo-González, R.; Méndez-Albores, A.; Villarreal-Barajas, T.; Aceves-Hernández, J.; Miranda-Ruvalcaba, R.; Nicolás-Vázquez, I. A theoretical study of 8-chloro-9-hydroxy-aflatoxin B1, the conversion product of aflatoxin B1 by neutral electrolyzed water. Toxins 2016, 225, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-González, R.; Bahena, L.; Tellez, J.L.A.; Torrés, J.H.; Ruvalcaba, R.M.; Hernández, J.M.A. Characterization and comparison of perezone with some analogues. Experimental and theoretical study. J. Mol. Struct. 2015, 1097, 98–105. [Google Scholar] [CrossRef]
- Jeong-Jeon, H.; Sook-Kim, J.; Gon-Kim, T.; Hyun-Kim, J.; Youk, J.H.; Yu, W. Preparation of poly(ε-caprolactone)-based polyurethane nanofibers containing silver nanoparticles. Appl. Surf. Sci. 2008, 254, 5886–5890. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I. A comprehensive review summarzing the effect of electrospinning paramteres and potential applications of nanofibers in biomedical an biotechnology. Arab. J. Chem. 2015, in press. [Google Scholar]
- Dias, J.R.; Antunes, F.E.; Bártolo, P.J. Influence of the Rheological Behaviour in Electrospun PCL Nanofibres Production for Tissue Engineering Applications. Chem. Eng. Trans. 2013, 32, 1015–1020. [Google Scholar]
- Tang, W.; Ng, S.-C. Facile synthesis of mono-6-amino-6-deoxy-α-, β-, γ-cyclodextrin hydrochlorides for molecular recognition, chiral separation and drug delivery. Nat. Protoc. 2008, 3, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, C.K.; Arseneau, S.; Blu, J.; Mauclaire, L.; Aychet, N. On the synthesis of new amphiphilic entities by the succinic coupling of β-cyclodextrins to calixarenes. Can. J. Chem. 2005, 83, 493–498. [Google Scholar] [CrossRef]
- Petter, R.C.; Salek, J.S.; Sikorski, C.T.; Kumaravel, G.; Lin, F. Cooperative Binding by Aggregated Mono-6-(alky1amino)-β-cyclodextrins. J. Am. Chem. Soc. 1990, 112, 3860–3868. [Google Scholar] [CrossRef]
- Muderawan, W.; Ong, T.T.; Lee, T.C.; Young, D.J.; Ching, C.B.; Ng, S.C. A reliable synthesis of 2- and 6-amino-β-cyclodextrin and permethylated-β-cyclodextrin. Tetrahedron Lett. 2005, 46, 7905–7907. [Google Scholar] [CrossRef]
- Rinehart, L. Fast atom bombardment masss spectrometry. Science 1982, 218, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.; Bordoli, R.S.; Sedwigck, R.D.; Tyler, A.N. Fast atom bombardment of solids as an ion soured in mass spectrometry. Nature 1981, 293, 270–275. [Google Scholar] [CrossRef]
- Shin, M.; Dong, T.; Inoue, Y. Morphological Change of Poly (ε-caprolactone) with a wide range of molecular weight via formation of inclusion complex with α-cylcodextrin. J. Polym. Sci. B Polym. Phys. 2005, 43, 1433–1440. [Google Scholar] [CrossRef]
- Shin, K.-M.; Dong, T.; He, Y.; Taguchi, Y.; Oishi, A.; Nishida, H.; Inoue, Y. Inclusion Complex Formation between α-Cyclodextrinand Biodegradable Aliphatic Polyesters. Macromol. Biosci. 2004, 4, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Gonil, P.; Sajomsang, W.; Ruktanonchai, U.R.; Pimpha, N.; Sramala, I.; Nuchuchua, O.; Saesoo, S.; Chaleawlert-umpon, S.; Puttipipatkhachorn, S. Novel quaternized chitosan containing β-cyclodextrin moiety: Synthesis, characterization and antimicrobial activity. Carbohydr. Polym. 2011, 83, 905–913. [Google Scholar] [CrossRef]
- Pessine, F.B.T.; Calderini, A.; Alexandrino, G.L. Review: Cyclodextrin Inclusion Complexes Probed by NMR Techniques. In Magnetic Resonance Spectroscopy; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Luo, H.; Fan, M.; Yu, Z.; Meng, X.; Li, B.; Zhang, S. Preparation and Properties of Degradable Shape Memory Material Based on Partial α-Cyclodextrin–Poly(ε-caprolactone) Inclusion complex. Macromol. Chem. Phys. 2009, 210, 669–676. [Google Scholar] [CrossRef]
- Narayanan, G.; Gupta, B.; Tonelli, A. Estimation of the poly(ε-caprolactone) [PCL] and α-cyclodextrin [α-CD] stoichiometric ratios in their inclusion complexes [ICs], and evaluation of porosity and fiber alignment in PCL nanofibers containing these ICs. Data Brief 2015, 5, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Nan, S.Y.; Fang, Z.Y.; Jun, Z.W. Preparation and Characterization of Inclusion Complex between β-Cyclodextrin and Polylactic Acid. Polymer (Korea) 2015, 39, 261–267. [Google Scholar] [CrossRef]
- Chávez-Huerta, A.; Colina-Rinconca, M.; Valbuena, A.C.; López, A. Obtención y caracterizaciòn de papel de quitosano. Rev. Iberoam. Polím. 2013, 13, 41–51. [Google Scholar]
- Zhao, S.P.; Xu, W.L. Thermo-sensitive hydrogels formed from photocrosslinkable polypseudorotoxanes consisting of β-Cyclodextrin and Pluronic F68/PCL. J. Polym. Res. 2010, 17, 503–510. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Ramakrishna, S.; Lim, C.T. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Heise, H.M.; Kuckuk, R.; Bereck, A.; Riegel, D. Infrared spectroscopy and Raman spectroscopy of cyclodextrin derivatives and their ferrocene inclusion complexes. Vib. Spectrosc. 2010, 53, 19–23. [Google Scholar] [CrossRef]
- Seda-Tıglı, R.; Merve-Kazaroglu, N.; Mavis, B.; Gümüsderelıoglu, M. Cellular Behavior on Epidermal Growth Factor (EGF)-Immobilized PCL/Gelatin Nanofibrous Scaffolds. J. Biomater. Sci. 2011, 22, 207–223. [Google Scholar] [CrossRef] [PubMed]
















© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyers-Montoya, E.; García-Casillas, P.; Vargas-Requena, C.; Escobedo-González, R.; Martel-Estrada, S.-A.; Martínez-Pérez, C.A. Polycaprolactone/Amino-β-Cyclodextrin Inclusion Complex Prepared by an Electrospinning Technique. Polymers 2016, 8, 395. https://doi.org/10.3390/polym8110395
Moyers-Montoya E, García-Casillas P, Vargas-Requena C, Escobedo-González R, Martel-Estrada S-A, Martínez-Pérez CA. Polycaprolactone/Amino-β-Cyclodextrin Inclusion Complex Prepared by an Electrospinning Technique. Polymers. 2016; 8(11):395. https://doi.org/10.3390/polym8110395
Chicago/Turabian StyleMoyers-Montoya, Edgar, Perla García-Casillas, Claudia Vargas-Requena, René Escobedo-González, Santos-Adriana Martel-Estrada, and Carlos A. Martínez-Pérez. 2016. "Polycaprolactone/Amino-β-Cyclodextrin Inclusion Complex Prepared by an Electrospinning Technique" Polymers 8, no. 11: 395. https://doi.org/10.3390/polym8110395