Progress and Prospect of Ion Imprinting Technology in Targeted Extraction of Lithium
Abstract
:1. Introduction

2. The Current Lithium Extraction Process and Its Advantages and Disadvantages
2.1. Lithium Ore Lithium Extraction Process
2.1.1. Acid Process
Sulfuric Acid Roasting Method
Fluorochemical Method
2.1.2. Alkali Process
Alkali Method
Mixed Alkali Method
2.1.3. Salt Process
Sulfate Roasting Method
Carbonate Roasting Method
Chlorination Roasting Method
Mixed Salt Roasting Method
Comparative Summary of Salt Processes
2.1.4. Pressure-Cooking Process
2.1.5. Summary of Lithium Resources from Ores
2.2. Salt Lake Brine Lithium Extraction Process
2.2.1. Chemical Precipitation Method
2.2.2. Solvent Extraction Method
2.2.3. Electrochemical Method
2.2.4. Membrane Method
2.2.5. Adsorption Method
2.2.6. Summary of Lithium Resources from Salt Lakes
2.3. Summary
3. Synthesis Method of Lithium Ion-Imprinted Materials and Related Introduction
3.1. System of Lithium Ion-Imprinted Materials
3.2. Quantum Chemical Computing for Lithium Ion-Imprinted Materials
3.3. Introduction to Conventional Methods
3.3.1. Synthesis Mechanism
3.3.2. Conventional Methods
3.4. Characterization of Lithium Ion-Imprinted Materials
3.5. Performance Evaluation of Lithium Ion-Imprinted Materials
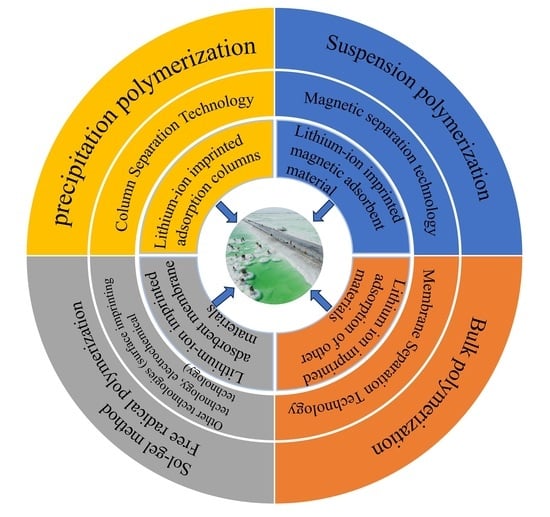
4. Different Types of Lithium Ion-Imprinted Materials
4.1. Lithium Ion-Imprinted Adsorption Columns
4.1.1. Preparation of Imprinted Adsorption Columns by Precipitation Polymerization Method
4.1.2. Preparation of Imprinted Adsorption Columns by Surface-Imprinted Polymerization Method
4.1.3. Electrochemical Preparation of Imprinted Adsorption Columns
4.2. Lithium-Ion Magnetically Imprinted Materials
Preparation of Imprinted Magnetic Adsorbent Materials by Surface-Imprinted Polymerization Method
4.3. Lithium Ion-Imprinted Membrane Materials
4.3.1. Preparation of Imprinted Adsorbent Membranes by Surface-Imprinted Polymerization Method
4.3.2. Electrochemical Preparation of Imprinted Adsorbent Membranes
4.3.3. Preparation of Imprinted Adsorbent Membranes by Hydrolysis Polymerization
4.4. Other Types of Lithium Ion-Imprinted Materials
4.4.1. Preparation of Imprinted Adsorbent Aerogels by Surface Imprint Polymerization
4.4.2. Functionalized Imprinted Polymer Brushes Prepared by UV-Initiated Surface Polymerization
4.4.3. Preparation of Imprinted Nanofibers by Electrochemical Methods
4.5. Summary of Materials
4.5.1. Advantages and Disadvantages of Lithium Ion-Imprinted Adsorption Columns
4.5.2. Advantages and Disadvantages of Lithium Ion Magnetic-Imprinted Materials
4.5.3. Advantages and Disadvantages of Lithium Ion-Imprinted Adsorbent Membrane Materials
5. Application of Lithium Ion-Imprinted Materials
5.1. Application of Lithium Ion-Imprinted Adsorption Columns
5.1.1. Application of Precipitation Polymerization Method for the Preparation of Imprinted Adsorption Columns
5.1.2. Application of Imprinted Adsorption Columns Prepared by Surface Imprint Polymerization Method
5.1.3. Application of Electrochemically Prepared Imprinted Adsorption Columns
5.2. Application of Lithium Ion Magnetic-Imprinted Materials
5.3. Application of Lithium Ion-Imprinted Membrane Materials
5.3.1. Application of Surface Imprint Polymerization for the Preparation of Imprinted Adsorbent Films
5.3.2. Application of Electrochemically Prepared Imprinted Adsorbent Membranes
5.3.3. Preparation of Imprinted Adsorbent Films using Hydrolysis Polymerization for Applications
5.4. Other Types of Materials for Lithium Ion
5.4.1. Application of Surface-Imprinted Polymerization for the Preparation of Imprinted Adsorbent Aerogels
5.4.2. Application of Functionalized Imprinted Polymer Brushes Prepared by UV-Initiated Surface Polymerization
5.4.3. Application of Electrochemical Methods for the Preparation of Nanofiber-Imprinted Materials
5.5. Application Summary
6. Summary and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Ma, B.; Lv, Y.; Wang, C.; Chen, Y. Thorough extraction of lithium and rubidium from lepidolite via thermal activation and acid leaching. Miner. Eng. 2022, 178, 107407. [Google Scholar] [CrossRef]
- Kuai, Y.; Yao, W.; Ma, H.; Liu, M.; Gao, Y.; Guo, R. Recovery lithium and potassium from lepidolite via potash calcination-leaching process. Miner. Eng. 2021, 160, 106643. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Rising to the Challenge: John B. Goodenough and Youngsik Kim, and “Challenges for Rechargeable Li Batteries”. Chem. Mater. 2015, 27, 5149–5150. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Merigot, K.; Rickard, W.D.A.; Evans, N.J.; McDonald, B.J.; Catovic, E.; Spitalny, P. Assessment of a spodumene ore by advanced analytical and mass spectrometry techniques to determine its amenability to processing for the extraction of lithium. Miner. Eng. 2018, 119, 137–148. [Google Scholar] [CrossRef]
- Karrech, A.; Azadi, M.R.; Elchalakani, M.; Shahin, M.A.; Seibi, A.C. A review on methods for liberating lithium from pegmatities. Miner. Eng. 2020, 145, 106085. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Ding, T.; Zheng, M.; Peng, S.; Lin, Y.; Zhang, X.; Li, M. Lithium extraction from salt lakes with different hydrochemical types in the Tibet Plateau. Geosci. Front. 2023, 14, 101485. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Li, J.; Kong, J.; Zhu, Q.; Li, H. In-situ capturing of fluorine with CaO for accelerated defluorination roasting of lepidolite in a fluidized bed reactor. Powder Technol. 2019, 353, 498–504. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, G.; Li, X.; Li, Y.; Wang, Z.; Chen, L. Electrolyte and current collector designs for stable lithium metal anodes. Int. J. Miner. Metall. Mater. 2022, 29, 953–964. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lü, Y.; Wang, C.; Chen, Y. A review of lithium extraction from natural resources. Int. J. Miner. Metall. Mater. 2023, 30, 209–224. [Google Scholar] [CrossRef]
- Rioyo, J.; Tuset, S.; Grau, R. Lithium Extraction from Spodumene by the Traditional Sulfuric Acid Process: A Review. Miner. Process. Extr. Metall. Rev. 2022, 43, 97–106. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Wang, Z.; Wu, X.; Wang, J.; Guo, H.; Hu, Q.; Peng, W. Extraction of lithium from lepidolite by sulfation roasting and water leaching. Int. J. Miner. Process. 2012, 110–111, 1–5. [Google Scholar] [CrossRef]
- Mulwanda, J.; Senanayake, G.; Oskierski, H.; Altarawneh, M.; Dlugogorski, B.Z. Leaching of lepidolite and recovery of lithium hydroxide from purified alkaline pressure leach liquor by phosphate precipitation and lime addition. Hydrometallurgy 2021, 201, 105538. [Google Scholar] [CrossRef]
- Xiao, C.; Zeng, L. Thermodynamic study on recovery of lithium using phosphate precipitation method. Hydrometallurgy 2018, 178, 283–286. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Z. Recovery of lithium from spent lithium-ion batteries using precipitation and electrodialysis techniques. Sep. Purif. Technol. 2018, 206, 335–342. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Sun, N.; Khoso, S.A.; Wang, L.; Sun, W. A novel precipitant for separating lithium from magnesium in high Mg/Li ratio brine. Hydrometallurgy 2019, 187, 125–133. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Fan, J.; Liu, X.; Hu, Y.; Hu, Y.; Zhou, Z.; Ren, Z. Recovery of Lithium Ions from Salt Lake Brine with a High Magnesium/Lithium Ratio Using Heteropolyacid Ionic Liquid. ACS Sustain. Chem. Eng. 2019, 7, 3062–3072. [Google Scholar] [CrossRef]
- Shi, C.; Jing, Y.; Jia, Y. Solvent extraction of lithium ions by tri-n-butyl phosphate using a room temperature ionic liquid. J. Mol. Liq. 2016, 215, 640–646. [Google Scholar] [CrossRef]
- Zhao, X.; Li, G.; Feng, M.; Wang, Y. Semi-continuous electrochemical extraction of lithium from brine using CF-NMMO/AC asymmetric hybrid capacitors. Electrochim. Acta 2020, 331, 135285. [Google Scholar] [CrossRef]
- Chen, Q.-B.; Ji, Z.-Y.; Liu, J.; Zhao, Y.-Y.; Wang, S.-Z.; Yuan, J.-S. Development of recovering lithium from brines by selective-electrodialysis: Effect of coexisting cations on the migration of lithium. J. Membr. Sci. 2018, 548, 408–420. [Google Scholar] [CrossRef]
- Gmar, S.; Chagnes, A. Recent advances on electrodialysis for the recovery of lithium from primary and secondary resources. Hydrometallurgy 2019, 189, 105124. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, R. Green recovery of lithium from water by a smart imprinted adsorbent with photo-controlled and selective properties. Chem. Eng. J. 2019, 378, 122084. [Google Scholar] [CrossRef]
- Mishra, S.; Verma, N. Surface ion imprinting-mediated carbon nanofiber-grafted highly porous polymeric beads: Synthesis and application towards selective removal of aqueous Pb(II). Chem. Eng. J. 2017, 313, 1142–1151. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Hassanpour, S. Selective adsorption of Cr(VI) ions from aqueous solutions using a Cr(VI)-imprinted polymer supported by magnetic multiwall carbon nanotubes. Polymer 2017, 132, 1–11. [Google Scholar] [CrossRef]
- Trzonkowska, L.; Leśniewska, B.; Godlewska-Żyłkiewicz, B. Studies on the effect of functional monomer and porogen on the properties of ion imprinted polymers based on Cr(III)-1,10-phenanthroline complex designed for selective removal of Cr(III) ions. React. Funct. Polym. 2017, 117, 131–139. [Google Scholar] [CrossRef]
- Fei, J.-J.; Wu, X.-H.; Sun, Y.-L.; Zhao, L.-Y.; Min, H.; Cui, X.-B.; Chen, Y.-J.; Liu, S.; Lian, H.-Z.; Li, C. Preparation of a novel amino functionalized ion-imprinted hybrid monolithic column for the selective extraction of trace copper followed by ICP-MS detection. Anal. Chim. Acta 2021, 1162, 338477. [Google Scholar] [CrossRef]
- Aljohani, M.S.; Alharbi, H.Y.; Monier, M. Development of an azo-functionalized ion-imprinted polymer for selective recognition of palladium ions. J. Clean. Prod. 2023, 426, 138966. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Yang, D.; Zheng, X.; Wang, Y.; Pan, J.; Zhang, T.; Xu, J.; Qiu, F.; Yan, Y.; et al. A facile strategy toward ion-imprinted hierarchical mesoporous material via dual-template method for simultaneous selective extraction of lithium and rubidium. J. Clean. Prod. 2018, 171, 264–274. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Liu, W.; Song, X.; Sun, S.; Fu, D.; Dong, G.; Wang, M.; Bai, Y.; Liu, X. Thermo-responsive ion imprinted polymer on the surface of magnetic carbon nanospheres for recognizing and capturing low-concentration lithium ion. Miner. Eng. 2023, 201, 108210. [Google Scholar] [CrossRef]
- Zhao, H.; Liang, Q.; Yang, Y.; Liu, W.; Liu, X. Magnetic graphene oxide surface lithium ion-imprinted material towards lithium extraction from salt lake. Sep. Purif. Technol. 2021, 265, 118513. [Google Scholar] [CrossRef]
- Yu, C.; Lu, J.; Dai, J.; Dong, Z.; Lin, X.; Xing, W.; Wu, Y.; Ma, Z. Bio-inspired fabrication of Ester-functionalized imprinted composite membrane for rapid and high-efficient recovery of lithium ion from seawater. J. Colloid Interface Sci. 2020, 572, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yue, X.; Wang, P.; Yu, T.; Du, X.; Hao, X.; Abudula, A.; Guan, G. Electrochemical technologies for lithium recovery from liquid resources: A review. Renew. Sustain. Energy Rev. 2022, 154, 111813. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, M.; Chen, X.; Zhao, Z. Separating lithium and magnesium in brine by aluminum-based materials. Hydrometallurgy 2018, 176, 73–77. [Google Scholar] [CrossRef]
- Han, Z.; Wu, S.; Wu, X.; Guan, W.; Cao, Z.; Li, Q.; Wang, M.; Zhang, G. Recycling of lithium and fluoride from LiF wastewater from LiF synthesis industry by solvent extraction. J. Environ. Chem. Eng. 2023, 11, 110557. [Google Scholar] [CrossRef]
- Chan, K.H.; Malik, M.; Azimi, G. Separation of lithium, nickel, manganese, and cobalt from waste lithium-ion batteries using electrodialysis. Resour. Conserv. Recycl. 2022, 178, 106076. [Google Scholar] [CrossRef]
- Luo, X.; Guo, B.; Luo, J.; Deng, F.; Zhang, S.; Luo, S.; Crittenden, J. Recovery of Lithium from Wastewater Using Development of Li Ion-Imprinted Polymers. ACS Sustain. Chem. Eng. 2015, 3, 460–467. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, W.; Liang, Q.; Liu, X.; Zhao, Z.; Yang, Y. Selective recovery of Li+ in acidic environment based on one novel electroactive Li+-imprinted graphene-based hybrid aerogel. Chem. Eng. J. 2020, 385, 123948. [Google Scholar] [CrossRef]
- Sun, D.; Meng, M.; Qiao, Y.; Zhao, Y.; Yan, Y.; Li, C. Synthesis of ion imprinted nanocomposite membranes for selective adsorption of lithium. Sep. Purif. Technol. 2018, 194, 64–72. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Y.; Wang, Y.; Ding, J.; Yu, P.; Yan, Y.; Li, C.; Zhou, Z. Fabrication of lithium ion imprinted hybrid membranes with antifouling performance for selective recovery of lithium. New J. Chem. 2018, 42, 118–128. [Google Scholar] [CrossRef]
- Liu, W.; Yan, G.; Zhang, E.; Liang, Q.; Qin, L.; Wang, M.; Liu, X.; Yang, Y. Extraction of lithium ions from acidic solution using electrochemically imprinted membrane. Desalination 2020, 496, 114751. [Google Scholar] [CrossRef]
- Han, N.; Li, Y.; Peng, H.; Gao, R.; He, Q.; Miao, Z.; Wan, K. Adsorption of Li+ by imprinted capacitor deionization—A new method for selective recovery of valuable lithium in acidic solutions. Desalination 2023, 565, 116820. [Google Scholar] [CrossRef]
- Yang, J.; Qu, G.; Liu, C.; Zhou, S.; Li, B.; Wei, Y. An effective lithium ion-imprinted membrane containing 12-crown ether-4 for selective recovery of lithium. Chem. Eng. Res. Des. 2022, 184, 639–650. [Google Scholar] [CrossRef]
- Kang, W.; Zhao, H.; Cui, Y.; Liu, X.; Yang, Y. Construction of novel stable surface ion-imprinted graphene aerogels for efficient and selective extraction of lithium ion. Sep. Purif. Technol. 2024, 333, 125946. [Google Scholar] [CrossRef]
- Bai, X.; Dai, J.; Ma, Y.; Bian, W.; Pan, J. 2-(Allyloxy) methylol-12-crown-4 ether functionalized polymer brushes from porous PolyHIPE using UV-initiated surface polymerization for recognition and recovery of lithium. Chem. Eng. J. 2020, 380, 122386. [Google Scholar] [CrossRef]
- Ding, T.; Wu, Q.; Nie, Z.; Zheng, M.; Wang, Y.; Yang, D. Selective recovery of lithium resources in salt lakes by polyacrylonitrile/ion-imprinted polymer: Synthesis, testing, and computation. Polym. Test. 2022, 113, 107647. [Google Scholar] [CrossRef]
- Rosales, G.D.; Pinna, E.G.; Suarez, D.S.; Rodriguez, M.H. Recovery Process of Li, Al and Si from Lepidolite by Leaching with HF. Minerals 2017, 7, 36. [Google Scholar] [CrossRef]
- Wang, H.-D.; Zhou, A.-A.; Guo, H.; Lü, M.-H.; Yu, H.-Z. Kinetics of leaching lithium from lepidolite using mixture of hydrofluoric and sulfuric acid. J. Cent. South Univ. 2020, 27, 27–36. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, G.; Li, H.; Pei, W.-t.; Wang, H.-d. Enhanced lithium leaching from lepidolite in continuous tubular reactor using H2SO4+H2SiF6 as lixiviant. Trans. Nonferr. Met. Soc. China 2021, 31, 2165–2173. [Google Scholar] [CrossRef]
- Guo, H.; Lv, M.; Kuang, G.; Cao, Y.; Wang, H. Stepwise heat treatment for fluorine removal on selective leachability of Li from lepidolite using HF/H2SO4 as lixiviant. Sep. Purif. Technol. 2021, 259, 118194. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, G.; Wan, H.; Yang, Y.; Yu, H.-Z.; Wang, H.-D. Enhanced acid treatment to extract lithium from lepidolite with a fluorine-based chemical method. Hydrometallurgy 2019, 183, 9–19. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Rohani, S.; He, M.; Tan, X.; Liu, W. Simultaneous extraction of lithium, rubidium, cesium and potassium from lepidolite via roasting with iron(II) sulfate followed by water leaching. Hydrometallurgy 2022, 208, 105820. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, H.; Wen, H.; Yang, Y. Lithium extraction from clay-type lithium resource using ferric sulfate solutions via an ion-exchange leaching process. Hydrometallurgy 2021, 206, 105759. [Google Scholar] [CrossRef]
- Yan, Q.-X.; Li, X.-H.; Wang, Z.-X.; Wang, J.-X.; Guo, H.-J.; Hu, Q.-Y.; Peng, W.-J.; Wu, X.-F. Extraction of lithium from lepidolite using chlorination roasting–water leaching process. Trans. Nonferr. Met. Soc. China 2012, 22, 1753–1759. [Google Scholar] [CrossRef]
- Zhang, X.; Aldahri, T.; Tan, X.; Liu, W.; Zhang, L.; Tang, S. Efficient co-extraction of lithium, rubidium, cesium and potassium from lepidolite by process intensification of chlorination roasting. Chem. Eng. Process.-Process Intensif. 2020, 147, 107777. [Google Scholar] [CrossRef]
- Luong, V.T.; Kang, D.J.; An, J.W.; Kim, M.J.; Tran, T. Factors affecting the extraction of lithium from lepidolite. Hydrometallurgy 2013, 134-135, 54–61. [Google Scholar] [CrossRef]
- Setoudeh, N.; Nosrati, A.; Welham, N.J. Lithium extraction from mechanically activated of petalite-Na2SO4 mixtures after isothermal heating. Miner. Eng. 2020, 151, 106294. [Google Scholar] [CrossRef]
- Vieceli, N.; Nogueira, C.A.; Pereira, M.F.C.; Durão, F.O.; Guimarães, C.; Margarido, F. Optimization of Lithium Extraction from Lepidolite by Roasting Using Sodium and Calcium Sulfates. Miner. Process. Extr. Metall. Rev. 2017, 38, 62–72. [Google Scholar] [CrossRef]
- Su, H.; Ju, J.; Zhang, J.; Yi, A.; Lei, Z.; Wang, L.; Zhu, Z.; Qi, T. Lithium recovery from lepidolite roasted with potassium compounds. Miner. Eng. 2020, 145, 106087. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, T.; He, L.; Zhao, Z.; Liu, X. A promising approach for directly extracting lithium from α-spodumene by alkaline digestion and precipitation as phosphate. Hydrometallurgy 2019, 189, 105141. [Google Scholar] [CrossRef]
- Grasso, M.L.; González, J.A.; Gennari, F.C. Lithium extraction from β-LiAlSi2O6 using Na2CO3 through thermal reaction. Miner. Eng. 2022, 176, 107349. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Q.; Chen, B.; Shi, X.; Liao, T. Preparation of lithium carbonate from spodumene by a sodium carbonate autoclave process. Hydrometallurgy 2011, 109, 43–46. [Google Scholar] [CrossRef]
- Santos, L.L.D.; Nascimento, R.M.D.; Pergher, S.B.C. Beta-spodumene:Na2CO3:NaCl system calcination: A kinetic study of the conversion to lithium salt. Chem. Eng. Res. Des. 2019, 147, 338–345. [Google Scholar] [CrossRef]
- An, J.W.; Kang, D.J.; Tran, K.T.; Kim, M.J.; Lim, T.; Tran, T. Recovery of lithium from Uyuni salar brine. Hydrometallurgy 2012, 117–118, 64–70. [Google Scholar] [CrossRef]
- Heidari, N.; Momeni, P. Selective adsorption of lithium ions from Urmia Lake onto aluminum hydroxide. Environ. Earth Sci. 2017, 76, 551. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Y.; Du, B.; Zhao, Y.; Wang, M. Recovery of both magnesium and lithium from high Mg/Li ratio brines using a novel process. Hydrometallurgy 2018, 175, 102–108. [Google Scholar] [CrossRef]
- Hai, C.; Zhou, Y.; Fuji, M.; Shirai, T.; Ren, X.; Zeng, J.; Li, X. Electrical conductivity of hydrothermally synthesized sodium lithium magnesium silicate. Mater. Res. Bull. 2018, 97, 473–482. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, R.; Wang, L.; Sun, W. Separation of magnesium from lithium in salt-lake brine through struvite precipitation. Miner. Eng. 2022, 180, 107468. [Google Scholar] [CrossRef]
- Shin, J.; Jeong, J.-M.; Lee, J.B.; Cho, H.-J.; Kim, Y.H.; Ryu, T. Preparation of lithium carbonate from waste lithium solution through precipitation and wet conversion methods. Hydrometallurgy 2022, 210, 105863. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.-S.; Zhang, W.; Ma, W.-C.; Li, Y.-P.; Chen, L.; Zhu, L.; Pan, Y. Recovering phosphorus and lithium separately from wastewater and brine using a novel coupled biofilm-precipitation system. J. Water Process Eng. 2023, 55, 104097. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. A Review on the Separation of Lithium Ion from Leach Liquors of Primary and Secondary Resources by Solvent Extraction with Commercial Extractants. Processes 2018, 6, 55. [Google Scholar] [CrossRef]
- Harvianto, G.R.; Kim, S.-H.; Ju, C.-S. Solvent extraction and stripping of lithium ion from aqueous solution and its application to seawater. Rare Met. 2016, 35, 948–953. [Google Scholar] [CrossRef]
- Zhou, Z.; Qin, W.; Fei, W. Extraction Equilibria of Lithium with Tributyl Phosphate in Three Diluents. J. Chem. Eng. Data 2011, 56, 3518–3522. [Google Scholar] [CrossRef]
- Su, H.; Li, Z.; Zhang, J.; Zhu, Z.; Wang, L.; Qi, T. Recovery of lithium from salt lake brine using a mixed ternary solvent extraction system consisting of TBP, FeCl3 and P507. Hydrometallurgy 2020, 197, 105487. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Liu, R.; Zhou, Y.; Zhang, Y.; Ji, L.; Li, L. Recovery of lithium from salt lake brine with high Na/Li ratio using solvent extraction. J. Mol. Liq. 2022, 362, 119667. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Yu, D.-H.; Jia, C.-Y.; Sun, L.-Y.; Tong, A.; Wang, Y.; Wang, Y.-X.; Huang, L.-J.; Tang, J.-G. Advances and promotion strategies of membrane-based methods for extracting lithium from brine. Desalination 2023, 566, 116891. [Google Scholar] [CrossRef]
- Arana Juve, J.-M.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for metal removal and recovery: A review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Culcasi, A.; Gurreri, L.; Cipollina, A.; Tamburini, A.; Micale, G. A comprehensive multi-scale model for bipolar membrane electrodialysis (BMED). Chem. Eng. J. 2022, 437, 135317. [Google Scholar] [CrossRef]
- Alkhadra, M.A.; Gao, T.; Conforti, K.M.; Tian, H.; Bazant, M.Z. Small-scale desalination of seawater by shock electrodialysis. Desalination 2020, 476, 114219. [Google Scholar] [CrossRef]
- Gao, S.-L.; Qin, Z.-X.; Wang, B.-F.; Huang, J.; Xu, Z.-L.; Tang, Y.-J. Lithium recovery from the spent lithium-ion batteries by commercial acid-resistant nanofiltration membranes: A comparative study. Desalination 2024, 572, 117142. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, B.; Wang, R. Insights into ion-imprinted materials for the recovery of metal ions: Preparation, evaluation and application. Sep. Purif. Technol. 2022, 298, 121469. [Google Scholar] [CrossRef]
- Hashemi, B.; Shamsipur, M.; Seyedzadeh, Z. Synthesis of ion imprinted polymeric nanoparticles for selective pre-concentration and recognition of lithium ions. New J. Chem. 2016, 40, 4803–4809. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, R. An efficient lithium ion imprinted adsorbent using multi-wall carbon nanotubes as support to recover lithium from water. J. Clean. Prod. 2018, 205, 201–209. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, L.; Li, H.; Guo, R.; Zhao, M.; Yang, L. Highly Selective Lithium Ion Adsorbents: Polymeric Porous Microsphere with Crown Ether Groups. Trans. Tianjin Univ. 2019, 25, 101–109. [Google Scholar] [CrossRef]
- Lu, J.; Qin, Y.; Zhang, Q.; Wu, Y.; Cui, J.; Li, C.; Wang, L.; Yan, Y. Multilayered ion-imprinted membranes with high selectivity towards Li+ based on the synergistic effect of 12-crown-4 and polyether sulfone. Appl. Surf. Sci. 2018, 427, 931–941. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Yang, D.; Zhang, T.; Qiu, F.; Pan, J. Calix [4]arenes functionalized dual-imprinted mesoporous film for the simultaneous selective recovery of lithium and rubidium. Appl. Organomet. Chem. 2018, 32, e4511. [Google Scholar] [CrossRef]
- Jo, H.; Le, T.-H.; Lee, H.; Lee, J.; Kim, M.; Lee, S.; Chang, M.; Yoon, H. Macrocyclic ligand-embedded graphene-in-polymer nanofiber membranes for lithium ion recovery. Chem. Eng. J. 2023, 452, 139274. [Google Scholar] [CrossRef]
- Li, Z.; He, G.; Zhao, G.; Niu, J.; Li, L.; Bi, J.; Mu, H.; Zhu, C.; Chen, Z.; Zhang, L.; et al. Preparation of a novel ion-imprinted membrane using sodium periodate-oxidized polydopamine as the interface adhesion layer for the direction separation of Li+ from spent lithium-ion battery leaching solution. Sep. Purif. Technol. 2021, 277, 119519. [Google Scholar] [CrossRef]
- Ren, Z.; Zhu, X.; Du, J.; Kong, D.; Wang, N.; Wang, Z.; Wang, Q.; Liu, W.; Li, Q.; Zhou, Z. Facile and green preparation of novel adsorption materials by combining sol-gel with ion imprinting technology for selective removal of Cu(II) ions from aqueous solution. Appl. Surf. Sci. 2018, 435, 574–584. [Google Scholar] [CrossRef]
- Shamsipur, M.; Besharati-Seidani, A.; Fasihi, J.; Sharghi, H. Synthesis and characterization of novel ion-imprinted polymeric nanoparticles for very fast and highly selective recognition of copper(II) ions. Talanta 2010, 83, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Jagirani, M.S.; Balouch, A.; Mahesar, S.A.; Kumar, A.; Baloch, A.R.; Abdullah; Bhanger, M.I. Fabrication of cadmium tagged novel ion imprinted polymer for detoxification of the toxic Cd2+ ion from aqueous environment. Microchem. J. 2020, 158, 105247. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.; Zhao, Q.; Hou, Y.; Chen, J. Ultrasensitive Quantum Dot Fluorescence quenching Assay for Selective Detection of Mercury Ions in Drinking Water. Sci. Rep. 2014, 4, 5624. [Google Scholar] [CrossRef]
- Zhou, Z.; Kong, D.; Zhu, H.; Wang, N.; Wang, Z.; Wang, Q.; Liu, W.; Li, Q.; Zhang, W.; Ren, Z. Preparation and adsorption characteristics of an ion-imprinted polymer for fast removal of Ni(II) ions from aqueous solution. J. Hazard. Mater. 2018, 341, 355–364. [Google Scholar] [CrossRef]
- Yin, F.; Mo, Y.; Liu, X.; Pang, Y.; Wu, X.; Hao, L.; Yu, J.; Xu, F. Surface-imprinted polymer microspheres for rapid and selective adsorption of As(V) ions from the aqueous phase. Mater. Chem. Phys. 2022, 281, 125687. [Google Scholar] [CrossRef]
- Lancet, D.; Pecht, I. Spectroscopic and immunochemical studies with nitrobenzoxadiazolealanine, a fluorescent dinitrophenyl analog. Biochemistry 1977, 16, 5150–5157. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jia, L.; Cheng, J.; Sun, Z. Magnetic ordered mesoporous carbon materials for adsorption of minocycline from aqueous solution: Preparation, characterization and adsorption mechanism. J. Hazard. Mater. 2019, 362, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.C.; Thavorn-Amornsri, T.; Pereira, M.F.R.; Figueiredo, J.L. Adsorption of ciprofloxacin on surface-modified carbon materials. Water Res. 2011, 45, 4583–4591. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, J.; Wang, Z.; Wang, Y.; Wang, S.; Yan, R.; Xu, Q. Highly-efficient and selective adsorption of anionic dyes onto hollow polymer microcapsules having a high surface-density of amino groups: Isotherms, kinetics, thermodynamics and mechanism. J. Colloid Interface Sci. 2019, 542, 123–135. [Google Scholar] [CrossRef]
- Vareda, J.P. On validity, physical meaning, mechanism insights and regression of adsorption kinetic models. J. Mol. Liq. 2023, 376, 121416. [Google Scholar] [CrossRef]
- Pandiarajan, A.; Kamaraj, R.; Vasudevan, S.; Vasudevan, S. OPAC (orange peel activated carbon) derived from waste orange peel for the adsorption of chlorophenoxyacetic acid herbicides from water: Adsorption isotherm, kinetic modelling and thermodynamic studies. Bioresour. Technol. 2018, 261, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Jin, S.; Lu, Q.; Ji, J. Adsorption isotherm, kinetic and mechanism of expanded graphite for sulfadiazine antibiotics removal from aqueous solutions. Environ. Technol. 2017, 38, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Deng, Y.; Merchant, A.; Su, J.; Zeng, G.; Long, X.; Zhong, M.-E.; Yang, L.; Gong, D.; Bai, L.; et al. Insights into Surface Ion-imprinted Materials for Heavy Metal Ion Treatment: Challenges and Opportunities. Sep. Purif. Rev. 2023, 52, 123–134. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, E.-H.; Yan, G.; Yang, Y.-Z.; Liu, W.-F.; Liu, X.-G. A lithium ion-imprinted adsorbent using magnetic carbon nanospheres as a support for the selective recovery of lithium ions. Carbon 2021, 176, 651. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, Y.; Meng, M.; Qiao, Y.; Yan, Y.; Li, C. Fabrication of highly selective ion imprinted macroporous membranes with crown ether for targeted separation of lithium ion. Sep. Purif. Technol. 2017, 175, 19–26. [Google Scholar] [CrossRef]











































| Project Name | Calculation Formula | Reference | |
|---|---|---|---|
| Adsorption capacity Qt (mg·g−1) | [31,32] | ||
| Isothermal adsorption model | Langmuir model | [96,97] | |
| Freundlich model | [98] | ||
| Tempkin model | [99] | ||
| Kinetic model | Quasi-primary dynamics | [100] | |
| Quasi-secondary dynamics | |||
| Separation factor RL value | [101] | ||
| Selective evaluation equations | Distribution factor Kd | [31,102] | |
| Selection factor α | |||
| Relative selection factor α’ | |||
| The Name of the Material | Type of Material | Synthesis Method | Template Ions | Complexing Agent | Functional Monomers | Cross-linker | Initiator | Carrier | Solvent Adsorption | Capacity | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Li/Rb-IHPS | Adsorption columns | Precipitation polymerization method | LiCl·H2O | 12C4 | MAA | EGDMA | AIBN | HPS | CH3CN | 2321.6 μg·g−1 | [30] |
| Li+-IIP | Imprinted adsorption columns | Surface-imprinted polymerization method | LiNO3 | DB14C4 | MAA | EGDMA | AIBN | MWCNT | DMSO | 32.23 μmol·g−1 | [84] |
| Fe3O4@SiO2@IIP | Magnetic imprinted material | Surface-imprinted polymerization method | LiCl·H2O | 2M12C4 | TEOS | EGDMA | AIBN | MH-Fe3O4@SiO2 | CH3OH and DMF | 0.586 mmol·g−1 | [38] |
| Fe3O4@C@IIP | Magnetic imprinted material | Surface-imprinted polymerization method | LiClO4 | B12C4 | NIPAM | EGDMA | AIBN | Fe3O4@C | CH3CN | 23.46 mg·g−1 | [31] |
| IIP-GO/Fe3O4@C | Magnetic imprinted material | Surface-imprinted polymerization method | LiClO4 | B12C4 | MAA | EGDMA | AIBN | GO/Fe3O4@C | DMF | 31.24 mg·L−1 | [32] |
| Li+-IIP-Fe3O4@C | Magnetic imprinted material | Surface-imprinted polymerization method | LiClO4 | 2M12C4 | MAA | EGDMA | AIBN | GO/Fe3O4@C | DMF | 31.24 mg·L−1 | [104] |
| IIMMs | Imprinted membrane material | Surface-imprinted polymerization method | LiCl | 2M12C4 | DA | EGDMA | AIBN | PVDF | CH3OH | 27.1 mg·g−1 | [105] |
| IINcMs | Imprinted membrane material | Surface-imprinted polymerization method | LiCl | 2M12C4 | PDA | EGDMA | AIBN | MPTS-Ag/PDA/PVDF | CH3OH | 25.58 mg·g−1 | [40] |
| LIIP@N-CMS/GA | Imprinted membrane material | Electrochemical | LiClO4 | B12C4 | GO | PPy | / | N-CMS/GA | KCl | 59.58 mg·g−1 | [39] |
| Imprinted membrane material | Imprinted membrane material | Electrochemical | LiCl | 2M12C4 | / | Py | / | / | KCl | 16.4 mg·g−1 | [42] |
| LIHMs | Imprinted membrane material | Hydrolysis polymerization method | LiCl | 12C4E | APTES | TEOS | / | PVDF | C2H5OH | 132 mg·g−1 | [44] |
| LIHMs | Imprinted membrane material | Hydrolysis polymerization method | LiCl | 12C4E | VTES | TEOS | / | pDA@GO/PVDF | C2H5OH | 27.10 mg·g−1 | [41] |
| IDGAs | Imprinted aerogel material | Precipitation polymerization method | LiClO4 | 2M12C4 | MAA | DGDMA | AIBN | DGA | C2H5OH | 11.50 mg·g−1 | [45] |
| The Name of the Material | Adsorption Capacity Range | Optimal pH | Number of Adsorption–Desorption | Remaining Adsorption Capacity | References |
|---|---|---|---|---|---|
| Li/Rb-IHPS | 0–200 μg·g−1 | 7 | 5 | 93% | [30] |
| Li+-IIP | 0–1400 μmol·g−1 | 6 | 10 | 89.7% | [84] |
| N@Li+-IPDI | 0–15 mg·g−1 | 6 | 5 | 93.42% | [43] |
| Fe3O4@SiO2@IIP | 0–1 mmol·g−1 | 6 | 5 | 92.4% | [38] |
| Li+-IIP | 0–30 mg·g−1 | / | 5 | 91.47% | [31] |
| IIP-GO/Fe3O4@C | 0–30 mg·g−1 | 6 | 6 | 91% | [32] |
| Li+-IIP-Fe3O4@C | 0–15 mg·g−1 | / | 6 | 92% | [104] |
| IIMMs | 20–200 mg·g−1 | 9 | 6 | 90.91% | [105] |
| IINcMs | 2–50 mg·g−1 | / | 10 | 92.10% | [40] |
| LIIP@N-CMS/GA | 0–60 mg·g−1 | 9 | 10 | 91.70% | [39] |
| Li+-IIM | 5–50 mg·g−1 | 7 | 5 | 95.88% | [42] |
| LIIMs | 0–200 mg·g−1 | / | 6 | 97% | [44] |
| LIHMs | 20–200 mg·g−1 | 6 | 6 | 91.80% | [41] |
| LIIP@N-CMS/GA | 10–200 mg·g−1 | 9 | 10 | 91.7% | [39] |
| LIIMs | 30–700 mg·g−1 | / | 6 | 97% | [44] |
| IDGAs | 0–15 mg·g−1 | / | 4 | 88.50% | [45] |
| polyHIPE | 0–5 mg·g−1 | 6 | 5 | 96.4% | [46] |
| IIP@SG/GO | 0–2 mg·g−1 | 8 | 5 | 89.09% | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, K.; Duan, J.; Zhang, J.; Huang, L.; Guo, L.; Wang, L. Progress and Prospect of Ion Imprinting Technology in Targeted Extraction of Lithium. Polymers 2024, 16, 833. https://doi.org/10.3390/polym16060833
Zhi K, Duan J, Zhang J, Huang L, Guo L, Wang L. Progress and Prospect of Ion Imprinting Technology in Targeted Extraction of Lithium. Polymers. 2024; 16(6):833. https://doi.org/10.3390/polym16060833
Chicago/Turabian StyleZhi, Keke, Jinwang Duan, Jiarui Zhang, Lianting Huang, Lianghui Guo, and Lulu Wang. 2024. "Progress and Prospect of Ion Imprinting Technology in Targeted Extraction of Lithium" Polymers 16, no. 6: 833. https://doi.org/10.3390/polym16060833