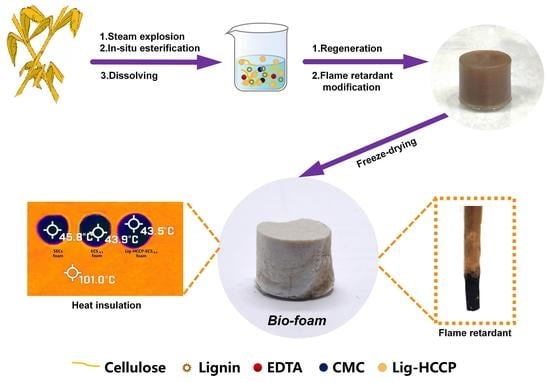
Preparation of Bio-Foam Material from Steam-Exploded Corn Straw by In Situ Esterification Modification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Esterification of Steam-Exploded Corn Straw
2.3. Preparation of the Bio-Foams from Steam-Exploded Corn Straw
2.4. Flame Retardant Modification of the Corn Straw Bio-Foam
2.5. Structure and Morphology Analysis
2.6. Thermal Performance Analysis
2.7. Compressive Mechanical Analysis
2.8. The Degree of Substitution (DS) Analysis
2.9. The Simulation Analysis Method with Finite Element Procedures
2.10. Characterization of Flame Retardancy
3. Results and Discussion
3.1. Characterization of Esterified Steam-Exploded Corn Straw
3.1.1. Morphological Characterization
3.1.2. Esterification Efficiency
3.1.3. The Chemical Structure Characterization
3.1.4. Thermal Properties
3.2. Morphology and Properties of the as-Prepared Corn Straw Bio-Foams
3.2.1. The Morphology
3.2.2. Mechanical Properties
3.2.3. Thermal Insulation
3.2.4. Flame Retardancy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandhu, R.S.; Shakya, M. Comparative study of synthetic plastics and biodegradable plastics. Glob. J. Bio-Sci. Biotechnol. 2019, 8, 107–112. [Google Scholar]
- Agustin-Salazar, S.; Ricciulli, M.; Ambrogi, V.; Cerruti, P.; Scarinzi, G. Effect of thermal annealing and filler ball-milling on the properties of highly filled polylactic acid/pecan nutshell biocomposites. Int. J. Biol. Macromol. 2022, 200, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Chiloeches, A.; Cuervo-Rodríguez, R.; López-Fabal, F.; Fernández-García, M.; Echeverría, C.; Muñoz-Bonilla, A. Antibacterial and compostable polymers derived from biobased itaconic acid as environmentally friendly additives for biopolymers. Polym. Test. 2022, 109, 107541. [Google Scholar] [CrossRef]
- Morici, E.; Carroccio, S.C.; Bruno, E.; Scarfato, P.; Filippone, G.; Dintcheva, N.T. Recycled (Bio)Plastics and (Bio)Plastic Composites: A Trade Opportunity in a Green Future. Polymers 2022, 14, 2038. [Google Scholar] [CrossRef]
- Guillaume, S.M. Sustainable and degradable plastics. Nat. Chem. 2022, 14, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Niranjana Prabhu, T.; Prashantha, K. A review on present status and future challenges of starch based polymer films and their composites in food packaging applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- do Val Siqueira, L.; Arias, C.I.L.F.; Maniglia, B.C.; Tadini, C.C. Starch-based biodegradable plastics: Methods of production, challenges and future perspectives. Curr. Opin. Food Sci. 2021, 38, 122–130. [Google Scholar] [CrossRef]
- Patil, S.; Bharimalla, A.K.; Mahapatra, A.; Dhakane-Lad, J.; Arputharaj, A.; Kumar, M.; Raja, A.S.M.; Kambli, N. Effect of polymer blending on mechanical and barrier properties of starch-polyvinyl alcohol based biodegradable composite films. Food Biosci. 2021, 44, 101352. [Google Scholar] [CrossRef]
- ChunYan, S.; Dong, L.; LiJun, W.; Yong, W. Biodegradation behavior and digestive properties of starch-based film for food packaging—A review. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Su, C.; Zhang, X.; Ge, X.; Shen, H.; Zhang, Q.; Lu, Y.; Sun, X.; Sun, Z.; Li, W. Structural, physical and degradation characteristics of polyvinyl alcohol/esterified mung bean starch/gliadin ternary composite plastic. Ind. Crops Prod. 2022, 176, 114365. [Google Scholar] [CrossRef]
- Zhou, X.; Cheng, R.; Wang, B.; Zeng, J.; Xu, J.; Li, J.; Kang, L.; Cheng, Z.; Gao, W.; Chen, K. Biodegradable sandwich-architectured films derived from pea starch and polylactic acid with enhanced shelf-life for fruit preservation. Carbohydr. Polym. 2021, 251, 117117. [Google Scholar] [CrossRef] [PubMed]
- Chikotsi, P.; Ncube, L.; Sibanda, N.; Ndlovu, L.; Nkomo, N. Development of Starch/Keratin/Chitosan Composite Material as a Substitute for Polystyrene in Food Packaging. J. Polym. Compos. 2019, 7, 1–7. [Google Scholar]
- Manker, L.P.; Jones, M.J.; Bertella, S.; Behaghel de Bueren, J.; Luterbacher, J.S. Current strategies for industrial plastic production from non-edible biomass. Curr. Opin. Green Sustain. Chem. 2023, 41, 100780. [Google Scholar] [CrossRef]
- Ghosh, K.; Jones, B.H. Roadmap to Biodegradable Plastics—Current State and Research Needs. ACS Sustain. Chem. Eng. 2021, 9, 6170–6187. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Arias, C.I.L.F.; Torres-Giner, S.; González-Martínez, C.; Chiralt, A. Valorization of Rice Straw into Cellulose Microfibers for the Reinforcement of Thermoplastic Corn Starch Films. Appl. Sci. 2021, 11, 8433. [Google Scholar] [CrossRef]
- Arpitha, G.R.; Verma, A.; Sanjay, M.R.; Gorbatyuk, S.; Khan, A.; Sobahi, T.R.; Asiri, A.M.; Siengchin, S. Bio-composite film from corn starch based vetiver cellulose. J. Nat. Fibers 2022, 19, 14634–14644. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Bajpai, S. Fabrication and characteristics of poly (vinyl alcohol)-starch-cellulosic material based biodegradable composite film for packaging application. Mater. Today Proc. 2020, 21 Pt 3, 1577–1582. [Google Scholar] [CrossRef]
- Sharma, M.; Beniwal, P.; Toor, A.P. The effect of rice straw derived microfibrillated cellulose as a reinforcing agent in starch/polyvinyl alcohol/polyethylene glycol biocompatible films. Mater. Chem. Phys. 2022, 291, 126652. [Google Scholar] [CrossRef]
- Rahmatika, A.M.; Goi, Y.; Kitamura, T.; Morita, Y.; Iskandar, F.; Ogi, T. Silica-supported carboxylated cellulose nanofibers for effective lysozyme adsorption: Effect of macropore size. Adv. Powder Technol. 2020, 31, 2932–2941. [Google Scholar] [CrossRef]
- Sun, Y.; Chu, Y.; Wu, W.; Xiao, H. Nanocellulose-based lightweight porous materials: A review. Carbohydr. Polym. 2021, 255, 117489. [Google Scholar] [CrossRef]
- Kryeziu, A.; Slovák, V.; Parchaňská, A. Liquefaction of Cellulose for Production of Advanced Porous Carbon Materials. Polymers 2022, 14, 1621. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Gu, J.; Jiang, F.; Hsieh, Y.-L. Holistic Rice Straw Nanocellulose and Hemicelluloses/Lignin Composite Films. ACS Sustain. Chem. Eng. 2016, 4, 728–737. [Google Scholar] [CrossRef]
- Asikainen, S.; Paakinaho, K.; Kyhkynen, A.-K.; Hannula, M.; Malin, M.; Ahola, N.; Kellomäki, M.; Seppälä, J. Hydrolysis and drug release from poly(ethylene glycol)-modified lactone polymers with open porosity. Eur. Polym. J. 2019, 113, 165–175. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, J.; Zhu, Y.; Zhang, J.; Luo, G.; Cao, P.; Shen, Q.; Zhang, L. Correlation Between the Structure and Compressive Property of PMMA Microcellular Foams Fabricated by Supercritical CO2 Foaming Method. Polymers 2020, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, Z.; Ou, B.; Liu, L.; Zhou, Z.; Shen, S.; Duan, Y. Design, preparation, and application of ordered porous polymer materials. Mater. Chem. Phys. 2014, 144, 213–225. [Google Scholar] [CrossRef]
- Zou, F.; Bouvard, J.-L.; Pradille, C.; Budtova, T. Ice-templated additive-free porous starches with tuned morphology and properties. Eur. Polym. J. 2022, 176, 111403. [Google Scholar] [CrossRef]
- Trovagunta, R.; Kelley, S.S.; Lavoine, N. Dual-Templating Approach for Engineering Strong, Biodegradable Lignin-Based Foams. ACS Sustain. Chem. Eng. 2022, 10, 15058–15067. [Google Scholar] [CrossRef]
- Mehra, S.; Nisar, S.; Chauhan, S.; Singh, G.; Singh, V.; Rattan, S. A dual stimuli responsive natural polymer based superabsorbent hydrogel engineered through a novel cross-linker. Polym. Chem. 2021, 12, 2404–2420. [Google Scholar] [CrossRef]
- Liu, X.; Huang, P.; Wang, J.; He, Y.; Song, P.; Wang, R. Preparation of porous biomass-based sponge with zein-alginate for oil absorption. Water Environ. J. 2022, 36, 704–712. [Google Scholar] [CrossRef]
- Schneider, W.D.H.; Dillon, A.J.P.; Camassola, M. Lignin nanoparticles enter the scene: A promising versatile green tool for multiple applications. Biotechnol. Adv. 2021, 47, 107685. [Google Scholar] [CrossRef]
- Solihat, N.N.; Hidayat, A.F.; Taib, M.N.A.M.; Hussin, M.H.; Lee, S.H.; Ghani, M.A.A.; Edrus, S.S.O.A.; Vahabi, H.; Fatriasari, W. Recent Developments in Flame-Retardant Lignin-Based Biocomposite: Manufacturing, and characterization. J. Polym. Environ. 2022, 30, 4517–4537. [Google Scholar] [CrossRef]
- Zhang, D.; Zeng, J.; Liu, W.; Qiu, X.; Qian, Y.; Zhang, H.; Yang, Y.; Liu, M.; Yang, D. Pristine lignin as a flame retardant in flexible PU foam. Green Chem. 2021, 23, 5972–5980. [Google Scholar] [CrossRef]
- Liang, X.; Hu, Q.; Wang, X.; Li, L.; Dong, Y.; Sun, C.; Hu, C.; Gu, X. Thermal Kinetics of a Lignin-Based Flame Retardant. Polymers 2020, 12, 2123. [Google Scholar] [CrossRef] [PubMed]
- Madyaratri, E.W.; Ridho, M.R.; Aristri, M.A.; Lubis, M.A.R.; Iswanto, A.H.; Nawawi, D.S.; Antov, P.; Kristak, L.; Majlingová, A.; Fatriasari, W. Recent Advances in the Development of Fire-Resistant Biocomposites—A Review. Polymers 2022, 14, 362. [Google Scholar]
- Dai, P.; Liang, M.; Ma, X.; Luo, Y.; He, M.; Gu, X.; Gu, Q.; Hussain, I.; Luo, Z. Highly Efficient, Environmentally Friendly Lignin-Based Flame Retardant Used in Epoxy Resin. ACS Omega 2020, 5, 32084–32093. [Google Scholar] [CrossRef]
- Chollet, B.; Lopez-Cuesta, J.-M.; Laoutid, F.; Ferry, L. Lignin Nanoparticles as A Promising Way for Enhancing Lignin Flame Retardant Effect in Polylactide. Materials 2019, 12, 2132. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Chen, M.-J.; Li, R.-M.; Zhang, X.-Q.; Feng, J.; Feng, J.; Liu, C.-F.; Shi, Q.-S. Homogeneous Transesterification of Sugar Cane Bagasse toward Sustainable Plastics. ACS Sustain. Chem. Eng. 2017, 5, 360–366. [Google Scholar] [CrossRef]
- Xia, Y.; Klinger, J.; Bhattacharjee, T.; Thompson, V. The elastoplastic flexural behaviour of corn stalks. Biosyst. Eng. 2022, 216, 218–228. [Google Scholar] [CrossRef]
- Dahmen, N. Pyrolysis of Biomass. Von S. Wang, Z. Luo. Chem. Ing. Tech. 2017, 89, 1255–1256. [Google Scholar] [CrossRef]
- Heredia, A.; Jiménez, A.; Guillén, R. Composition of plant cell walls. Z. Lebensm. Unters. Forsch. 1995, 200, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Vorwerk, S.; Somerville, S.; Somerville, C. The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 2004, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Rehman, N.; de Miranda, M.I.G.; Rosa, S.M.L.; Pimentel, D.M.; Nachtigall, S.M.B.; Bica, C.I.D. Cellulose and Nanocellulose from Maize Straw: An Insight on the Crystal Properties. J. Polym. Environ. 2014, 22, 252–259. [Google Scholar] [CrossRef]
- Ge, J.; Li, F.; Gao, Y.; Jin, J.; Jiang, W. A high-performance structural material based on maize straws and its biodegradable composites of poly (propylene carbonate). Cellulose 2021, 28, 11381–11395. [Google Scholar] [CrossRef]
- Liubimtsev, N.A.; Deniz, A.; Elmanovich, I.V.; Gallyamov, M.O.; Pich, A. Morphology and Properties of Flame-Retardant Superhydrophobic Polymer Coatings Deposited on Cotton Fabrics from Supercritical CO2. ACS Appl. Polym. Mater. 2020, 2, 2919–2926. [Google Scholar] [CrossRef]
- Rhili, K.; Chergui, S.; ElDouhaibi, A.S.; Siaj, M. Hexachlorocyclotriphosphazene Functionalized Graphene Oxide as a Highly Efficient Flame Retardant. ACS Omega 2021, 6, 6252–6260. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Structure and properties of high quality natural cellulose fibers from cornstalks. Polymer 2005, 46, 5494–5500. [Google Scholar] [CrossRef]
- Ye, D.; Rongpipi, S.; Kiemle, S.N.; Barnes, W.J.; Chaves, A.M.; Zhu, C.; Norman, V.A.; Liebman-Peláez, A.; Hexemer, A.; Toney, M.F.; et al. Preferred crystallographic orientation of cellulose in plant primary cell walls. Nat. Commun. 2020, 11, 4720. [Google Scholar] [CrossRef]
- Schestakow, M.; Karadagli, I.; Ratke, L. Cellulose aerogels prepared from an aqueous zinc chloride salt hydrate melt. Carbohydr. Polym. 2016, 137, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Baillis, D.; Coquard, R.; Moura, L.M. Heat transfer in cellulose-based aerogels: Analytical modelling and measurements. Energy 2015, 84, 732–744. [Google Scholar] [CrossRef]
- Xu, M.-J.; Xu, G.-R.; Leng, Y.; Li, B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym. Degrad. Stab. 2016, 123, 105–114. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Peng, X.; Zhong, L.; Sun, R.; Jiang, D. Rapid Synthesis of Cellulose Esters by Transesterification of Cellulose with Vinyl Esters under the Catalysis of NaOH or KOH in DMSO. J. Agric. Food Chem. 2013, 61, 2489–2495. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Wei, L.; Hu, H.; Huang, Z.; Yang, M.; Huang, A.; Wu, J.; Feng, Z. Esterification mechanism of lignin with different catalysts based on lignin model compounds by mechanical activation-assisted solid-phase synthesis. RSC Advances 2017, 7, 52382–52390. [Google Scholar] [CrossRef]







| Temperature (°C) | Time (min) | Amount of VA (mol) | |
|---|---|---|---|
| k1 * | 4.42 | 4.29 | 3.80 |
| k2 * | 4.23 | 4.15 | 4.29 |
| k3 * | 4.06 | 4.49 | 4.67 |
| k4 * | 4.59 | 4.37 | 4.54 |
| SS * | 0.313 | 0.284 | 1.184 |
| Mean square | 0.105 | 0.094 | 0.395 |
| Optimal level | A4 | B3 | C3 |
| Influence effect | C (Amount of VA) > A (Temperature) > B (Time) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Zhou, Y.; Du, X.; Xu, W.; Lu, Y.; Wang, F.; Jiang, M. Preparation of Bio-Foam Material from Steam-Exploded Corn Straw by In Situ Esterification Modification. Polymers 2023, 15, 2222. https://doi.org/10.3390/polym15092222
Pan Y, Zhou Y, Du X, Xu W, Lu Y, Wang F, Jiang M. Preparation of Bio-Foam Material from Steam-Exploded Corn Straw by In Situ Esterification Modification. Polymers. 2023; 15(9):2222. https://doi.org/10.3390/polym15092222
Chicago/Turabian StylePan, Yu, Yufan Zhou, Xiaoqing Du, Wangjie Xu, Yuan Lu, Feng Wang, and Man Jiang. 2023. "Preparation of Bio-Foam Material from Steam-Exploded Corn Straw by In Situ Esterification Modification" Polymers 15, no. 9: 2222. https://doi.org/10.3390/polym15092222