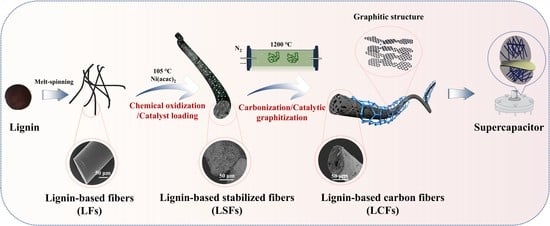
Fabrication of Graphitized Carbon Fibers from Fusible Lignin and Their Application in Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Melt Spinning of PEGL
2.3. Preparation of CFs from PEGL
2.4. Supercapacitor Assembly
2.5. Characterization
2.5.1. Morphological Analysis
2.5.2. Thermal Analysis
2.5.3. Carbonaceous Structure Analysis
2.5.4. Electrochemical Performance Measurements
3. Results and Discussion
3.1. Morphological and Thermal Analysis
3.2. Structural Determination
3.3. Electrochemical Performance of the LCF-Based Supercapacitors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.; Chen, S.; Ni, Y. Current Overview of Carbon Fiber: Toward Green Sustainable Raw Materials. BioResources 2020, 15, 7234–7259. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, W.; Jia, D.; Cui, L.; Liu, J. Thermally-treated and acid-etched carbon fiber cloth based on pre-oxidized polyacrylonitrile as self-standing and high area-capacitance electrodes for flexible supercapacitors. Chem. Eng. J. 2019, 364, 70–78. [Google Scholar] [CrossRef]
- Wang, S.; Bai, J.; Tendo, I.M.; Wang, Q.; Xiang, H.; Tang, J.; Zhu, M. Lignin-based carbon fibers: Formation, modification and potential applications. Green Energy Environ. 2022, 7, 578–605. [Google Scholar] [CrossRef]
- Li, Q.; Hu, C.; Li, M.; Truong, P.; Li, J.; Lin, H.-S.; Naik, M.T.; Xiang, S.; Jackson, B.E.; Kuo, W.; et al. Enhancing the multi-functional properties of renewable lignin carbon fibers via defining the structure–property relationship using different biomass feedstocks. Green Chem. 2021, 23, 3725–3739. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, C.; Zhang, X.; Yang, C.; Jiang, M.; Zhang, X. A sustainable platform of lignin: From bioresources to materials and their applications in rechargeable batteries and supercapacitors. Prog. Energy Combust. Sci. 2020, 76, 100788.1–100788.24. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.O.; Duque-Brito, E.; Villafan-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Bengtsson, A.; Hecht, P.; Sommertune, J.; Ek, M.; Sedin, M.; Sjöholm, E. Carbon Fibers from Lignin–Cellulose Precursors: Effect of Carbonization Conditions. ACS Sustain. Chem. Eng. 2020, 8, 6826–6833. [Google Scholar] [CrossRef]
- Sun, S.-C.; Xu, Y.; Wen, J.-L.; Yuan, T.-Q.; Sun, R. Recent advances of lignin-based carbon fibers (LCFs): Precursors, fabrications, properties, and applications. Green Chem. 2022, 24, 5709–5738. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Xia, C.; Wang, H.-F.; Tang, C. Recent advances in electrocatalytic oxygen reduction for on-site hydrogen peroxide synthesis in acidic media. J. Energy Chem. 2022, 67, 432–450. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Sevastyanova, O. Carbon Materials from Technical Lignins: Recent Advances. Top. Curr. Chem. 2018, 376, 33. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Contescu, C.I.; Liu, P.; Wang, S.; Lee, S.-H.; Guo, J.; Young, T.M. Understanding the local structure of disordered carbons from cellulose and lignin. Wood Sci. Technol. 2021, 55, 587–606. [Google Scholar] [CrossRef]
- Yan, Q.; Arango, R.; Li, J.; Cai, Z. Fabrication and characterization of carbon foams using 100% Kraft lignin. Mater. Des. 2021, 201, 109460. [Google Scholar] [CrossRef]
- Wang, D.; Lee, S.H.; Kim, J.; Park, C.B. “Waste to Wealth”: Lignin as a Renewable Building Block for Energy Harvesting/Storage and Environmental Remediation. ChemSusChem 2020, 13, 2807–2827. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Liu, K.; Zhang, M.; Liu, W.; Li, H.; Du, H.; Si, C. Lignin-based electrodes for energy storage application. Ind. Crops Prod. 2021, 165, 113425. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Yan, G.; Li, X.; Wang, J. High-Value Utilization of Lignin To Prepare Functional Carbons toward Advanced Lithium-Ion Capacitors. ACS Sustain. Chem. Eng. 2020, 8, 11522–11531. [Google Scholar] [CrossRef]
- Luo, Y.; Qu, W.; Cochran, E.; Bai, X. Enabling high-quality carbon fiber through transforming lignin into an orientable and melt-spinnable polymer. J. Clean. Prod. 2021, 307, 127252. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, D.; Ding, W.P.; Zhu, Z.Z.; Wang, G.Z.; He, J.R.; Wang, H.B.; Fei, P.; Si, T.L. Promising Rice-Husk-Derived Carbon/Ni(OH)2 Composite Materials as a High-Performing Supercapacitor Electrode. ACS Omega 2020, 5, 29896–29902. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, S.; Zhu, M.; Liu, Y. Novel silicon-contained lignin-based carbon fibers derived from bamboo pulping black liquor with improved electrochemical performance for supercapacitors. J. Appl. Polym. Sci. 2021, 138, 51321. [Google Scholar] [CrossRef]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.-P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High surface area activated carbon from rice husk as a high performance supercapacitor electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef]
- Chen, W.; Wang, X.; Feizbakhshan, M.; Liu, C.; Hong, S.; Yang, P.; Zhou, X. Preparation of lignin-based porous carbon with hierarchical oxygen-enriched structure for high-performance supercapacitors. J. Colloid Interface Sci. 2019, 540, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zhu, M.; Chen, J.; Song, Y.; Li, Y.; Zhou, J. Novel Lignin-Cellulose-Based Carbon Nanofibers as High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2019, 12, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Ko, F.K.; Renneckar, S. Impact of Thermal Oxidative Stabilization on the Performance of Lignin-Based Carbon Nanofiber Mats. ACS Omega 2019, 4, 5345–5355. [Google Scholar] [CrossRef] [PubMed]
- Shokrani Havigh, R.; Mahmoudi Chenari, H. A comprehensive study on the effect of carbonization temperature on the physical and chemical properties of carbon fibers. Sci. Rep. 2022, 12, 10704. [Google Scholar] [CrossRef]
- Pignol, G.; Bassil, P.; Fontmorin, J.M.; Floner, D.; Geneste, F.; Hapiot, P. Electrochemical Properties of Carbon Fibers from Felts. Molecules 2022, 27, 6584. [Google Scholar] [CrossRef]
- Park, J.; Kwac, L.K.; Kim, H.G.; Shin, H.K. Carbon Papers from Tall Goldenrod Cellulose Fibers and Carbon Nanotubes for Application as Electromagnetic Interference Shielding Materials. Molecules 2022, 27, 1842. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Q.; Zhai, G.; Xiang, H.; Zhou, J.; Jia, C.; Zhu, L.; Wu, Q.; Zhu, M. Continuously processing waste lignin into high-value carbon nanotube fibers. Nat. Commun. 2022, 13, 5755. [Google Scholar] [CrossRef]
- Schierholz, R.; Kroger, D.; Weinrich, H.; Gehring, M.; Tempel, H.; Kungl, H.; Mayer, J.; Eichel, R.A. The carbonization of polyacrylonitrile-derived electrospun carbon nanofibers studied by in situ transmission electron microscopy. RSC Adv. 2019, 9, 6267–6277. [Google Scholar] [CrossRef]
- Novák, I.; Šauša, O.; Maťko, I.; Simsek Bilgin, E.; Berek, D. Microporous carbon fibers prepared by carbonization of cellulose as carriers of particles of active substances. Chem. Pap. 2019, 74, 1359–1365. [Google Scholar] [CrossRef]
- Maťko, I.; Šauša, O.; Čechová, K.; Novák, I.; Švajdlenková, H.; Berek, D.; Pecz, M. Porous carbon fibers prepared from cellulose Characterization of microstructure and water sorption properties. J. Therm. Anal. Calorim. 2019, 138, 1997–2004. [Google Scholar] [CrossRef]
- Yang, J.; Nakabayashi, K.; Miyawaki, J.; Yoon, S.-H. Preparation of pitch based carbon fibers using Hyper-coal as a raw material. Carbon 2016, 106, 28–36. [Google Scholar] [CrossRef]
- Dasari, B.L.; Nouri, J.M.; Brabazon, D.; Naher, S. Graphene and derivatives–Synthesis techniques, properties and their energy applications. Energy 2017, 140, 766–778. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Schnepp, Z.; Ramirez-Rico, J. Structural Evolution in Iron-Catalyzed Graphitization of Hard Carbons. Chem. Mater. 2021, 33, 3087–3097. [Google Scholar] [CrossRef]
- Geng, L.; Cai, Y.; Lu, L.; Zhang, Y.; Li, Y.; Chen, B.; Peng, X.-F. Highly Strong and Conductive Carbon Fibers Originated from Bioinspired Lignin/Nanocellulose Precursors Obtained by Flow-Assisted Alignment and In Situ Interfacial Complexation. ACS Sustain. Chem. Eng. 2021, 9, 2591–2599. [Google Scholar] [CrossRef]
- Wang, K.; Cao, Y.; Wang, X.; Kharel, P.R.; Gibbons, W.; Luo, B.; Gu, Z.; Fan, Q.; Metzger, L. Nickel catalytic graphitized porous carbon as electrode material for high performance supercapacitors. Energy 2016, 101, 9–15. [Google Scholar] [CrossRef]
- Sagues, W.J.; Jain, A.; Brown, D.; Aggarwal, S.; Suarez, A.; Kollman, M.; Park, S.; Argyropoulos, D.S. Are lignin-derived carbon fibers graphitic enough? Green Chem. 2019, 21, 4253–4265. [Google Scholar] [CrossRef]
- Destyorini, F.; Yudianti, R.; Irmawati, Y.; Hardiansyah, A.; Hsu, Y.-I.; Uyama, H. Temperature driven structural transition in the nickel-based catalytic graphitization of coconut coir. Diam. Relat. Mater. 2021, 117, 108443. [Google Scholar] [CrossRef]
- Chang, B.; Guo, Y.; Li, Y.; Yin, H.; Zhang, S.; Yang, B.; Dong, X. Graphitized hierarchical porous carbon nanospheres: Simultaneous activation/graphitization and superior supercapacitance performance. J. Mater. Chem. A 2015, 3, 9565–9577. [Google Scholar] [CrossRef]
- Kubo, S.; Uraki, Y.; Sano, Y. Catalytic graphitization of hardwood acetic acid lignin with nickel acetate. J. Wood Sci. 2003, 49, 188–192. [Google Scholar] [CrossRef]
- Choi, B.-K.; Choi, W.-K.; Seo, M.-K. Effect of catalytic graphitization on the electric heating performance of electroless nickel-coated carbon fibers. Curr. Appl. Phys. 2022, 42, 86–91. [Google Scholar] [CrossRef]
- Takahashi, S.; Nge, T.T.; Takata, E.; Ohashi, Y.; Yamada, T. Flocculation properties of polyethylene glycol-modified lignin. Sep. Purif. Technol. 2020, 253, 117524. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, J.; Zhao, G. Preparation and Characterization of Modified Soda Lignin with Polyethylene Glycol. Materials 2016, 9, 822. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Duan, J.; Koda, K.; Yamada, T.; Uraki, Y. Preparation of electric double layer capacitors (EDLCs) from two types of electrospun lignin fibers. Holzforschung 2016, 70, 661–671. [Google Scholar] [CrossRef]
- Torres-Canas, F.; Bentaleb, A.; Fӧllmer, M.; Roman, J.; Neri, W.; Ly, I.; Derré, A.; Poulin, P. Improved structure and highly conductive lignin-carbon fibers through graphene oxide liquid crystal. Carbon 2020, 163, 120–127. [Google Scholar] [CrossRef]
- Sun, Z.; Yao, D.; Cao, C.; Zhang, Z.; Zhang, L.; Zhu, H.; Yuan, Q.; Yi, B. Preparation and formation mechanism of biomass-based graphite carbon catalyzed by iron nitrate under a low-temperature condition. J. Environ. Manag. 2022, 318, 115555. [Google Scholar] [CrossRef]
- Sun, X.; Jin, H.; Qu, W. Lignin-derived 3D porous graphene on carbon cloth for flexible supercapacitors. RSC Adv. 2021, 11, 19695–19704. [Google Scholar] [CrossRef]
- You, X.; Misra, M.; Gregori, S.; Mohanty, A.K. Preparation of an Electric Double Layer Capacitor (EDLC) Using Miscanthus-Derived Biocarbon. ACS Sustain. Chem. Eng. 2017, 6, 318–324. [Google Scholar] [CrossRef]
- Luo, W.; Guo, N.; Wang, L.; Cao, Y.; Xu, M.; Jia, D.; Feng, S.; Gong, X.; Zhang, S. From powders to freestanding electrodes: Assembly active particles into bacterial cellulose for high performance supercapacitors. Electrochim. Acta 2021, 387, 138560. [Google Scholar] [CrossRef]
- You, X.; Koda, K.; Yamada, T.; Uraki, Y. Preparation of electrode for electric double layer capacitor from electrospun lignin fibers. Holzforschung 2015, 69, 1097–1106. [Google Scholar] [CrossRef]
- Zhi, M.; Liu, S.; Hong, Z.; Wu, N. Electrospun activated carbon nanofibers for supercapacitor electrodes. RSC Adv. 2014, 4, 43619–43623. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.; Zhang, H.; Shen, M.; Baker, F.; Liu, Y.; Liu, L.; Yang, J.; Cao, H.; Xu, Q.; et al. Houttuynia-derived nitrogen-doped hierarchically porous carbon for high-performance supercapacitor. Carbon 2020, 161, 62–70. [Google Scholar] [CrossRef]
- Adam, A.A.O.D.J.; Al-Hadeethi, Y.; Mkawi, E.M.; Abubakar Abdulkadir, B.U.F.; Mudassir Hassan, Y.; Wadi, I.A.S.M. State of the Art and New Directions on Electrospun Lignin/Cellulose Nanofibers for Supercapacitor Application: A Systematic Literature Review. Polymers 2020, 12, 2884. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kwon, J.H.; Lee, J.-W.; Lee, J.H.; Roh, K.C. An effective approach to preparing partially graphitic activated carbon derived from structurally separated pitch pine biomass. Carbon 2017, 118, 431–437. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, W.; Yin, E.; Yu, S.; Wang, S.; Xiang, H.; Li, D.; Zhu, M. Melt Spinning of Low-Cost Activated Carbon Fiber with a Tunable Pore Structure for High-Performance Flexible Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 9360–9368. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Liu, X.; Li, L. Biomass derived interconnected hierarchical micro-meso-macro- porous carbon with ultrahigh capacitance for supercapacitors. Carbon 2019, 147, 540–549. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, Y.; Zhang, L.; Xie, Y.; Ye, X. Facile synthesis of three-dimensional porous carbon for high-performance supercapacitors. J. Alloy. Compd. 2019, 787, 1–8. [Google Scholar] [CrossRef]
- Lin, J.; Kubo, S.; Yamada, T.; Koda, K.; Uraki, Y. Chemical thermostabilization for the preparation of carbon fibers from softwood lignin. Bioresources 2012, 7, 5634–5646. [Google Scholar] [CrossRef]
- Malik, M.; Chan, K.H.; Azimi, G. Quantification of nickel, cobalt, and manganese concentration using ultraviolet-visible spectroscopy. RSC Adv. 2021, 11, 28014–28028. [Google Scholar] [CrossRef]
- Yoo, S.; Chung, C.-C.; Kelley, S.S.; Park, S. Graphitization Behavior of Loblolly Pine Wood Investigated byin SituHigh Temperature X-ray Diffraction. ACS Sustain. Chem. Eng. 2018, 6, 9113–9119. [Google Scholar] [CrossRef]
- Gutiérrez-Pardo, A.; Ramírez-Rico, J.; Cabezas-Rodríguez, R.; Martínez-Fernández, J. Effect of catalytic graphitization on the electrochemical behavior of wood derived carbons for use in supercapacitors. J. Power Sources 2015, 278, 18–26. [Google Scholar] [CrossRef]
- Inagaki, M.; Kang, F. Materials Science and Engineering of Carbon: Fundamentals; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Gai, L.; Li, J.; Wang, Q.; Tian, R.; Li, K. Evolution of biomass to porous graphite carbon by catalytic graphitization. J. Environ. Chem. Eng. 2021, 9, 106678. [Google Scholar] [CrossRef]
- Li, T.; Ma, R.; Xu, X.; Sun, S.; Lin, J. Microwave-induced preparation of porous graphene nanosheets derived from biomass for supercapacitors. Microporous Mesoporous Mater. 2021, 324, 111277. [Google Scholar] [CrossRef]
- Xia, S.; Cai, N.; Wu, J.; Xiao, H.; Hu, J.; Chen, X.; Chen, Y.; Yang, H.; Wang, X.; Chen, H. Synthesis and formation mechanism of biomass-based mesoporous graphitic carbon. Fuel Process. Technol. 2020, 209, 106543. [Google Scholar] [CrossRef]
- Kim, J.; Kang, J.G.; Choi, J.; Braun, P.V.; Kim, S.-K. Fiber Electrodes Mesostructured on Carbon Fibers for Energy Storage. ACS Appl. Energy Mater. 2021, 4, 13716–13724. [Google Scholar] [CrossRef]
- Mei, B.; Munteshari, O.; Lau, J.; Dunn, B.S.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Vicentini, R.; Aguiar, J.P.; Beraldo, R.; Venâncio, R.; Rufino, F.; Da Silva, L.M.; Zanin, H. Ragone Plots for Electrochemical Double-Layer Capacitors. Batter. Supercaps 2021, 4, 1291–1303. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer. Science 2006, 313, 1760–3. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Lou, F.; Melandsø Buan, M.E.; Sheridan, E.; Chen, D. Enhancing capacitance of supercapacitor with both organic electrolyte and ionic liquid electrolyte on a biomass-derived carbon. RSC Adv. 2017, 7, 23859–23865. [Google Scholar] [CrossRef]
- Du, B.; Chai, L.; Zhu, H.; Cheng, J.; Wang, X.; Chen, X.; Zhou, J.; Sun, R.C. Effective fractionation strategy of sugarcane bagasse lignin to fabricate quality lignin-based carbon nanofibers supercapacitors. Int. J. Biol. Macromol. 2021, 184, 604–617. [Google Scholar] [CrossRef]
- Schlee, P.; Hosseinaei, O.; Baker, D.; Landmér, A.; Tomani, P.; Mostazo-López, M.J.; Cazorla-Amorós, D.; Herou, S.; Titirici, M.-M. From Waste to Wealth: From Kraft Lignin to Free-standing Supercapacitors. Carbon 2019, 145, 470–480. [Google Scholar] [CrossRef]
- Dai, Z.; Ren, P.-G.; Jin, Y.-L.; Zhang, H.; Ren, F.; Zhang, Q. Nitrogen-sulphur Co-doped graphenes modified electrospun lignin/polyacrylonitrile-based carbon nanofiber as high performance supercapacitor. J. Power Sources 2019, 437, 226937. [Google Scholar] [CrossRef]
- Poochai, C.; Srikhaow, A.; Lohitkarn, J.; Kongthong, T.; Tuantranont, S.; Tuantranont, S.; Primpray, V.; Maeboonruan, N.; Wisitsoraat, A.; Sriprachuabwong, C. Waste coffee grounds derived nanoporous carbon incorporated with carbon nanotubes composites for electrochemical double-layer capacitors in organic electrolyte. J. Energy Storage 2021, 43, 103169. [Google Scholar] [CrossRef]
- Wu, J.; Xia, M.; Zhang, X.; Chen, Y.; Sun, F.; Wang, X.; Yang, H.; Chen, H. Hierarchical porous carbon derived from wood tar using crab as the template: Performance on supercapacitor. J. Power Sources 2020, 455, 227982. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, G.; Li, Y.; Yao, Y.; Liu, Q.; Xing, B.; Jia, J.; Zhang, C. Structural evolution of porous graphitic carbon nanosheets based on quinonyl decomposition for supercapacitor electrodes. Appl. Surf. Sci. 2021, 537, 147824. [Google Scholar] [CrossRef]
- Zeng, C.; Duan, C.; Guo, Z.; Liu, Z.; Dou, S.; Yuan, Q.; Liu, P.; Zhang, J.; Luo, J.; Liu, W.; et al. Ultrafastly activated needle coke as electrode material for supercapacitors. Prog. Nat. Sci. Mater. Int. 2022, 32, 786–792. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, T.; Jiang, C. Highly mesoporous carbon flakes derived from a tubular biomass for high power electrochemical energy storage in organic electrolyte. Mater. Chem. Phys. 2019, 223, 16–23. [Google Scholar] [CrossRef]







| Sample | Extrapolated Onset Decomposition Temperature (Tod, °C) | Extrapolated Endset Decomposition Temperature (Ted, °C) | Mass Loss at Decomposition (%) | Mass Loss at Carbonization and Graphitization (%) | Total Mass Loss (%) |
|---|---|---|---|---|---|
| LSF-0 | 312.7 | 471.7 | 39.4 | 14.1 | 53.5 |
| LSF-0.2 | 309.1 | 468.4 | 41.2 | 13.3 | 54.5 |
| LSF-0.4 | 308.4 | 467.9 | 43.9 | 12.5 | 56.4 |
| LSF-0.6 | 289.5 | 463.5 | 45.7 | 12.4 | 58.1 |
| Sample | Raman | XRD | ||
|---|---|---|---|---|
| R = ID/IG | La (nm) | Lc (nm) | d002 (nm) | |
| LCF-0 | 8.16 | 2.36 | – | – |
| LCF-0.2 | 3.74 | 5.14 | 0.26 | 0.37 |
| LCF-0.4 | 2.57 | 7.48 | 0.41 | 0.35 |
| LCF-0.6 | 3.71 | 5.18 | 0.39 | 0.37 |
| Samples | BET a (m2 g−1) | Wp b (nm) | Vtotal c (m3 g−1) | Sinternal d (m2 g−1) | Sexternal e (m2 g−1) |
|---|---|---|---|---|---|
| LCF-0 | 350.95 | 1.62 | 0.13 | 316.67 | 34.28 |
| LCF-0.2 | 103.52 | 1.65 | 0.04 | 93.96 | 9.57 |
| LCF-0.4 | 89.92 | 1.61 | 0.03 | 83.74 | 6.19 |
| LCF-0.6 | 269.56 | 1.67 | 0.11 | 234.48 | 35.08 |
| Sample | CCV a (F g−1) | CGCD b (F g−1) | Rs c (Ω) | IR d (V) | E e (Wh kg−1) | P f (W kg−1) |
|---|---|---|---|---|---|---|
| LCF-0 | 1.3 | 0.6 | 6.3 | 0.10 | 0.8 | 767.9 |
| LCF-0.2 | 16.3 | 8.7 | 2.0 | 0.07 | 11.7 | 811.6 |
| LCF-0.4 | 22.0 | 10.7 | 1.2 | 0.05 | 15.7 | 869.5 |
| LCF-0.6 | 18.9 | 9.6 | 21.0 | 0.08 | 13.2 | 829.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; You, X.; Wang, L.; Qi, S.; Wang, R.; Uraki, Y.; Zhang, H. Fabrication of Graphitized Carbon Fibers from Fusible Lignin and Their Application in Supercapacitors. Polymers 2023, 15, 1947. https://doi.org/10.3390/polym15081947
Zhou L, You X, Wang L, Qi S, Wang R, Uraki Y, Zhang H. Fabrication of Graphitized Carbon Fibers from Fusible Lignin and Their Application in Supercapacitors. Polymers. 2023; 15(8):1947. https://doi.org/10.3390/polym15081947
Chicago/Turabian StyleZhou, Linfei, Xiangyu You, Lingjie Wang, Shijie Qi, Ruichen Wang, Yasumitsu Uraki, and Huijie Zhang. 2023. "Fabrication of Graphitized Carbon Fibers from Fusible Lignin and Their Application in Supercapacitors" Polymers 15, no. 8: 1947. https://doi.org/10.3390/polym15081947