A New Phosphorous/Nitrogen-Containing Flame-Retardant Film with High Adhesion for Jute Fiber Composites
Abstract
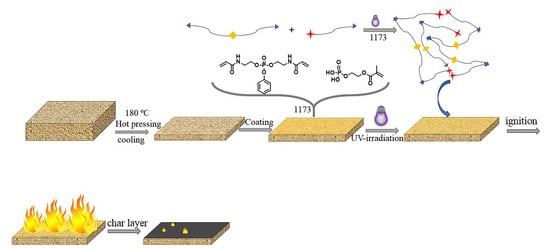
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Phosphorus Nitrogen Flame-Retardant Monomer (PDHAA)
2.3. Preparation of Phosphorus Nitrogen Flame-Retardant Monomer (PDHAA)
2.4. Characterization
3. Results and Discussion
3.1. Structural Characterization of PDHAA Monomer
3.2. Curing Behavior of UV-Curable Coatings
3.3. Flame Retardancy of the Fiber Composites
3.4. SEM Analysis of Char Morphology
3.5. Thermal Properties of the Fiber Composites
3.6. Raman Spectroscopic Characterization of Carbon Residues
3.7. Flame-Retardant Mode of Action of the UV-Curable Coatings
3.8. Mechanical Properties of the FNFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serra-Parareda, F.; Tarres, Q.; Espinach, F.X.; Vilaseca, F.; Mutje, P.; Delgado-Aguilar, M. Influence of lignin content on the intrinsic modulus of natural fibers and on the stiffness of composite materials. Int. J. Biol. Macromol. 2020, 155, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Shalwan, A.; Yousif, B.F. In State of Art: Mechanical and tribological behaviour of polymeric composites based on natural fibres. Mater. Des. 2013, 48, 14–24. [Google Scholar] [CrossRef]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M.S. A Review on Natural Fiber Reinforced Polymer Composite and Its Applications. Int. J. Polym. Sci. 2015, 2015, 243947. [Google Scholar] [CrossRef]
- Zhao, W.J.; Hu, Q.X.; Zhang, N.N.; Wei, Y.C.; Zhao, Q.; Zhang, Y.M.; Dong, J.B.; Sun, Z.Y.; Liu, B.J.; Li, L.; et al. In situ inorganic flame retardant modified hemp and its polypropylene composites. RSC Adv. 2017, 7, 32236–32245. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, T.; Zhang, W.; Dou, Y. Influence of surface flame-retardant layer containing ammonium polyphosphate and expandable graphite on the performance of jute/polypropylene composites. J. Therm. Anal. Calorim. 2018, 135, 2367–2375. [Google Scholar] [CrossRef]
- Baştürk, E.; Oktay, B.; Kahraman, M.V.; Kayaman Apohan, N. UV cured thiol-ene flame retardant hybrid coatings. Prog. Org. Coat. 2013, 76, 936–943. [Google Scholar] [CrossRef]
- Naik, D.; Wazarkar, K.; Sabnis, A. UV-curable flame-retardant coatings based on phosphorous and silicon containing oligomers. J. Coat. Technol. Res. 2018, 16, 733–743. [Google Scholar] [CrossRef]
- Chambhare, S.U.; Lokhande, G.P.; Jagtap, R.N. Design and UV-curable behaviour of boron based reactive diluent for epoxy acrylate oligomer used for flame retardant wood coating. Des. Monomers Polym. 2017, 20, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Xing, W.; Zhang, P.; Song, L.; Hu, Y. Functionalization of Cotton with UV-Cured Flame Retardant Coatings. Ind. Eng. Chem. Res. 2012, 51, 5394–5401. [Google Scholar] [CrossRef]
- Xing, W.; Jie, G.; Song, L.; Hu, S.; Lv, X.; Wang, X.; Hu, Y. Flame retardancy and thermal degradation of cotton textiles based on UV-curable flame retardant coatings. Thermochim. Acta 2011, 513, 75–82. [Google Scholar] [CrossRef]
- Liang, B.; Hong, X.; Zhu, M.; Gao, C.; Wang, C.; Tsubaki, N. Synthesis of novel intumescent flame retardant containing phosphorus, nitrogen and boron and its application in polyethylene. Polym. Bull. 2015, 72, 2967–2978. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.; Hu, Y. Synthesis of a novel flame retardant containing phosphorus, nitrogen and boron and its application in flame-retardant epoxy resin. Polym. Degrad. Stab. 2016, 133, 358–366. [Google Scholar] [CrossRef]
- Terekhov, I.V.; Chistyakov, E.M.; Filatov, S.N.; Deev, I.S.; Kurshev, E.V.; Lonskii, S.L. Factors Influencing the Fire-Resistance of Epoxy Compositions Modified with Epoxy-Containing Phosphazenes. Inorg. Mater. Appl. Res. 2019, 10, 1429–1435. [Google Scholar] [CrossRef]
- Orlov, A.; Konstantinova, A.; Korotkov, R.; Yudaev, P.; Mezhuev, Y.; Terekhov, I.; Gurevich, L.; Chistyakov, E. Epoxy Compositions with Reduced Flammability Based on DER-354 Resin and a Curing Agent Containing Aminophosphazenes Synthesized in Bulk Isophoronediamine. Polymers 2022, 14, 3592. [Google Scholar] [CrossRef] [PubMed]
- Rybyan, A.A.; Bilichenko, J.V.; Kireev, V.V.; Kolenchenko, A.A.; Chistyakov, E.M. Curing of DER-331 Epoxy Resin with Arylaminocyclotriphosphazenes Based on o-, m-, and p-methylanilines. Polymers 2022, 14, 5334. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Cheng, L.; Wang, M. Preparation and flame retardancy of an intumescent flame-retardant epoxy resin system constructed by multiple flame-retardant compositions containing phosphorus and nitrogen heterocycle. Polym. Degrad. Stab. 2015, 119, 251–259. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Zhang, M.; Yuan, G. Synergistic and compatibilizing effect of octavinyl polyhedral oligomeric silsesquioxane nanoparticles in polypropylene/intumescent flame retardant composite system. Compos. Part A Appl. Sci. Manuf. 2019, 123, 46–58. [Google Scholar] [CrossRef]
- Chen, X.; Jiao, C.; Li, S.; Hu, Y. Preparation and properties of a single molecule intumescent flame retardant. Fire Saf. J. 2013, 58, 208–212. [Google Scholar] [CrossRef]
- Jiang, S.; Shi, Y.; Qian, X.; Zhou, K.; Xu, H.; Lo, S.; Gui, Z.; Hu, Y. Synthesis of a Novel Phosphorus- and Nitrogen-Containing Acrylate and Its Performance as an Intumescent Flame Retardant for Epoxy Acrylate. Ind. Eng. Chem. Res. 2013, 52, 17442–17450. [Google Scholar] [CrossRef]
- Lokhande, G.; Chambhare, S.; Jagtap, R. Synthesis and properties of phosphate-based diacrylate reactive diluent applied to UV-curable flame-retardant wood coating. J. Coat. Technol. Res. 2016, 14, 255–266. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Yang, X.; Zhang, Q.; Zheng, Y.; Ren, Y.; Cheng, B. A phosphorous/nitrogen-containing flame retardant with UV-curing for polyester/cotton fabrics. Cellulose 2022, 29, 1263–1281. [Google Scholar] [CrossRef]
- Beyler-Çiğil, A. Designing superhydrophobic and flame retardant photo-cured hybrid coatings. Prog. Org. Coat. 2020, 148, 105850. [Google Scholar] [CrossRef]
- Xing, W.; Song, L.; Jie, G.; Lv, X.; Wang, X.; Hu, Y. Synthesis and thermal behavior of a novel UV-curable transparent flame retardant film and phosphorus-nitrogen synergism of flame retardancy. Polym. Adv. Technol. 2011, 22, 2123–2129. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, K.; Hua, C.; Guo, X. Conformation Variation and Tunable Protein Adsorption through Combination of Poly(acrylic acid) and Antifouling Poly(N-(2-hydroxyethyl) acrylamide) Diblock on a Particle Surface. Polymers 2020, 12, 566. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, W.; Yu, D. Durable flame retardant finishing of cotton fabrics with halogen-free organophosphonate by UV photoinitiated thiol-ene click chemistry. Carbohydr. Polym. 2017, 172, 275–283. [Google Scholar] [CrossRef]
- Chen, L.; Song, L.; Lv, P.; Jie, G.; Tai, Q.; Xing, W.; Hu, Y. A new intumescent flame retardant containing phosphorus and nitrogen: Preparation, thermal properties and application to UV curable coating. Prog. Org. Coat. 2011, 70, 59–66. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Xing, W.; Lu, H.; Hu, Y. A effective flame retardant for epoxy resins based on poly(DOPO substituted dihydroxyl phenyl pentaerythritol diphosphonate). Mater. Chem. Phys. 2011, 125, 536–541. [Google Scholar] [CrossRef]
- Jiao, Z.; Yang, Q.; Wang, X.; Wang, C. UV-curable hyperbranched urethane acrylate oligomers modified with different fatty acids. Polym. Bull. 2017, 74, 5049–5063. [Google Scholar] [CrossRef]
- Li, X.; Bian, F.; Hu, J.; Li, S.; Gui, X.; Lin, S. One-step synthesis of novel multifunctional silicone acrylate prepolymers for use in UV-curable coatings. Prog. Org. Coat. 2022, 163, 106601. [Google Scholar] [CrossRef]
- Mali, P.; Sonawane, N.; Patil, V.; Mawale, R.; Pawar, N. Designing efficient UV-curable flame-retardant and antimicrobial wood coating: Phosphorodiamidate reactive diluent with epoxy acrylate. J. Polym. Res. 2021, 28, 376. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, S.; Liang, R.; Liao, Z.; You, G. A green highly-effective surface flame-retardant strategy for rigid polyurethane foam: Transforming UV-cured coating into intumescent self-extinguishing layer. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105534. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Y.; Wang, S.; Shaghaleh, H.; Sun, P.; Huang, X.; Xu, X.; Wang, S.; Liu, H. Flame-retarded polyurethane foam conferred by a bio-based nitrogen-phosphorus-containing flame retardant. React. Funct. Polym. 2021, 168, 105057. [Google Scholar] [CrossRef]
- Li, M.-E.; Yan, Y.-W.; Zhao, H.-B.; Jian, R.-K.; Wang, Y.-Z. A facile and efficient flame-retardant and smoke-suppressant resin coating for expanded polystyrene foams. Compos. Part B Eng. 2020, 185, 105057. [Google Scholar] [CrossRef]
- Wu, F.; Bao, X.; Wang, J. Flame Retardancy and Thermal Degradation Behaviors of Thiol-Ene Composites Containing a Novel Phosphorus and Silicon-Containing Flame Retardant. Polymers 2022, 14, 820. [Google Scholar] [CrossRef]
- Li, W.; Dou, Y.; Li, X.; Fang, S.; Li, J.; Li, Q. A Highly Effective, UV-Curable, Intumescent, Flame-Retardant Coating Containing Phosphorus, Nitrogen, and Sulfur, Based on Thiol-Ene Click Reaction. Materials 2022, 15, 3358. [Google Scholar] [CrossRef]
- Yao, C.; Xing, W.; Ma, C.; Song, L.; Hu, Y.; Zhuang, Z. Synthesis of phytic acid-based monomer for UV-Cured coating to improve fire safety of PMMA. Prog. Org. Coat. 2020, 140, 105497. [Google Scholar] [CrossRef]
- Wen, P.; Wang, X.; Feng, X.; Zhou, K.; Yu, B.; Zhang, Q.; Tai, Q.; Song, L.; Hu, Y.; Yuen, R.K.K. A novel UV-curing flame retardant film with significantly intumescent effect. Polym. Degrad. Stab. 2015, 119, 288–294. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Ghanbari, D.; Loghman-Estarki, M.R. Star-shaped PbS nanocrystals prepared by hydrothermal process in the presence of thioglycolic acid. Polyhedron 2012, 35, 149–153. [Google Scholar] [CrossRef]
- Phalak, G.; Patil, D.; Patil, A.; Mhaske, S. Synthesis of acrylated cardanol diphenyl phosphate for UV curable flame-retardant coating application. Eur. Polym. J. 2019, 121, 109320. [Google Scholar] [CrossRef]
- Dai, K.; Deng, Z.; Liu, G.; Wu, Y.; Xu, W.; Hu, Y. Effects of a Reactive Phosphorus-Sulfur Containing Flame-Retardant Monomer on the Flame Retardancy and Thermal and Mechanical Properties of Unsaturated Polyester Resin. Polymers 2020, 12, 1441. [Google Scholar] [CrossRef]
- Liu, J.; Dong, C.; Zhang, Z.; Sun, H.; Kong, D.; Lu, Z. Durable flame retardant cotton fabrics modified with a novel silicon–phosphorus–nitrogen synergistic flame retardant. Cellulose 2020, 27, 9027–9043. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Chiang, T.H.; Hsieh, T.E. A study of monomer’s effect on adhesion strength of UV-curable resins. Int. J. Adhes. Adhes. 2006, 26, 520–531. [Google Scholar] [CrossRef]












| Samples | PDHAA (g) | PM-2 (g) | LOI (%) | HBR (mm/min) or Self-Extinguishing Time(s) | UL-94 |
|---|---|---|---|---|---|
| FNFs | 21.6 | 14.63 mm/min | No rating | ||
| FNFs-1 | 0 | 6 | 26.4 | 65 s | No rating |
| FNFs-2 | 2 | 4 | 27.3 | 15 s | No rating |
| FNFs-3 | 3 | 3 | 27.5 | 9 s | No rating |
| FNFs-4 | 4 | 2 | 27.8 | 4 s | V-0 |
| FNFs-5 | 6 | 0 | 28.2 | 0 s | V-0 |
| Samples | PHRR (kW/m2) | Av-HRR (kW/m2) | THR (MJ/m2) | TSR (m2/m2) | TSP (m2) | Av-COY (kg/kg) | Av-CO2Y (kg/kg) |
|---|---|---|---|---|---|---|---|
| FNFs | 568.4 | 211.8 | 72.3 | 372.8 | 3.3 | 0.275 | 7.9 |
| FNFs-1 | 428.6 | 163.1 | 79.3 | 564.5 | 5.0 | 0.040 | 2.0 |
| FNFs-3 | 339.6 | 122.4 | 74.2 | 754.8 | 6.8 | 0.024 | 1.0 |
| FNFs-5 | 397.5 | 157.2 | 71.8 | 577.9 | 5.1 | 0.013 | 0.9 |
| Sample | Adhesion Level | Tensile Strength (MPa) |
|---|---|---|
| FNFs | 16.60 ± 1.09 | |
| FNFs-1 | 2 | 19.16 ± 1.28 |
| FNFs-2 | 3 | 26.19 ± 2.35 |
| FNFs-3 | 3 | 24.03 ± 0.14 |
| FNFs-4 | 4 | 24.85 ± 2.08 |
| FNFs-5 | 20.63 ± 1.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, Y.; Zhong, Z.; Huang, J.; Ju, A.; Yao, W.; Zhang, C.; Guan, D. A New Phosphorous/Nitrogen-Containing Flame-Retardant Film with High Adhesion for Jute Fiber Composites. Polymers 2023, 15, 1920. https://doi.org/10.3390/polym15081920
Dou Y, Zhong Z, Huang J, Ju A, Yao W, Zhang C, Guan D. A New Phosphorous/Nitrogen-Containing Flame-Retardant Film with High Adhesion for Jute Fiber Composites. Polymers. 2023; 15(8):1920. https://doi.org/10.3390/polym15081920
Chicago/Turabian StyleDou, Yanli, Zheng Zhong, Jiaming Huang, Aixun Ju, Weiguo Yao, Chunling Zhang, and Dongbo Guan. 2023. "A New Phosphorous/Nitrogen-Containing Flame-Retardant Film with High Adhesion for Jute Fiber Composites" Polymers 15, no. 8: 1920. https://doi.org/10.3390/polym15081920