Electrospun Fibrous Nanocomposite Sensing Materials for Monitoring Biomarkers in Exhaled Breath
Abstract
:1. Introduction
2. Materials and Methods
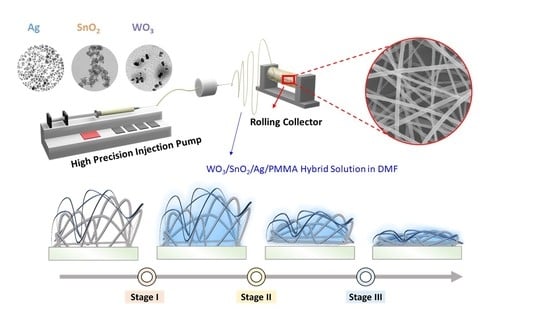
2.1. Preparation of Freestanding WO3/SnO2/Ag/PMMA Sensing Materials
2.2. Material Characterization
2.3. Optical Measurement of Extinction Spectrum
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, Y.; Shu, Z.; Zhou, J.; Li, T.; Wang, W.; Zhao, Z. One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl. Catal. B 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Victor, F.M.; de Lima Andrade, S.R.; Bandeira, F. Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic State. In Endocrinology and Diabetes: A Problem Oriented Approach; Bandeira, F., Gharib, H., Griz, L., Faria, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 355–362. [Google Scholar]
- Chreitah, A.; Hijazia, K.; Doya, L.; Salloum, A. Severe dyslipidemia associated with diabetic ketoacidosis in newly diagnosed female of type 1 diabetes mellitus. Oxf. Med. Case Rep. 2021, 2021, omab036. [Google Scholar] [CrossRef] [PubMed]
- Elgenidy, A.; Awad, A.K.; Saad, K.; Atef, M.; El-Leithy, H.H.; Obiedallah, A.A.; Hammad, E.M.; Ahmad, F.-A.; Ali, A.M.; Dailah, H.G.; et al. Incidence of diabetic ketoacidosis during COVID-19 pandemic: A meta-analysis of 124,597 children with diabetes. Pediatr. Res. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Kempf, K.; Röhling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef] [PubMed]
- McMichael, L.E.; Heath, H.; Johnson, C.M.; Fanter, R.; Alarcon, N.; Quintana-Diaz, A.; Pilolla, K.; Schaffner, A.; Jelalian, E.; Wing, R.R.; et al. Metabolites involved in purine degradation, insulin resistance, and fatty acid oxidation are associated with prediction of Gestational diabetes in plasma. Metabolomics 2021, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, Y. The Most Common Methods for Breath Acetone Concentration Detection: A Review. IEEE Sens. J. 2021, 21, 14540–14558. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Pang, A.L.; Ardani, M.R.; Pung, S.-Y.; Ooi, P.C.; Hamzah, A.A.; Mohd Razip Wee, M.F.; Aniq Shazni Mohammad Haniff, M.; Dee, C.F.; Mahmoudi, E.; et al. Detection of breath acetone by semiconductor metal oxide nanostructures-based gas sensors: A review. Mater. Sci. Semicond. Process. 2022, 149, 106897. [Google Scholar] [CrossRef]
- Güntner, A.T.; Weber, I.C.; Schon, S.; Pratsinis, S.E.; Gerber, P.A. Monitoring rapid metabolic changes in health and type-1 diabetes with breath acetone sensors. Sens. Actuators B Chem. 2022, 367, 132182. [Google Scholar] [CrossRef]
- Verma, A.; Yadav, D.; Singh, A.; Gupta, M.; Thapa, K.B.; Yadav, B.C. Detection of acetone via exhaling human breath for regular monitoring of diabetes by low-cost sensing device based on perovskite BaSnO3 nanorods. Sens. Actuators B Chem. 2022, 361, 131708. [Google Scholar] [CrossRef]
- Li, A.J.; Pal, V.K.; Kannan, K. A review of environmental occurrence, toxicity, biotransformation and biomonitoring of volatile organic compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Yan, H.; Zhou, Y.-G. Electrical sensing of volatile organic compounds in exhaled breath for disease diagnosis. Curr. Opin. Electrochem. 2022, 33, 100922. [Google Scholar] [CrossRef]
- Das, S.; Pal, M. Review—Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Prasanth, A.; Meher, S.R.; Alex, Z.C. Metal oxide thin films coated evanescent wave based fiber optic VOC sensor. Sens. Actuators A Phys. 2022, 338, 113459. [Google Scholar] [CrossRef]
- Mohar, M. 2,4,7-Triaminofluorenone as a Multi-Analyte Colorimetric Sensor of Fluoride, Acetone Vapor, and Other Harmful Compounds. ChemistrySelect 2019, 4, 8061–8067. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Sotelo Rios, S.E.; Agarwal, V. Simple one step synthesis of dual-emissive heteroatom doped carbon dots for acetone sensing in commercial products and Cr (VI) reduction. Chem. Eng. J. 2021, 414, 128830. [Google Scholar] [CrossRef]
- Kumar, A.; Pawar, D.; Late, D.J.; Kanawade, R. PVA-coated miniaturized flexible fiber optic sensor for acetone detection: A prospective study for non-invasive diabetes diagnosis. J. Mater. Sci. Mater. Electron. 2022, 33, 2509–2517. [Google Scholar] [CrossRef]
- Vadivel, S.; Balaji, G.; Rathinavel, S. High performance ethanol and acetone gas sensor based nanocrystalline MnCo2O4 using clad-modified fiber optic gas sensor. Opt. Mater. 2018, 85, 267–274. [Google Scholar] [CrossRef]
- Masikini, M.; Chowdhury, M.; Nemraoui, O. Review—Metal Oxides: Application in Exhaled Breath Acetone Chemiresistive Sensors. J. Electrochem. Soc. 2020, 167, 037537. [Google Scholar] [CrossRef] [Green Version]
- Broza, Y.Y.; Vishinkin, R.; Barash, O.; Nakhleh, M.K.; Haick, H. Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation. Chem. Soc. Rev. 2018, 47, 4781–4859. [Google Scholar] [CrossRef]
- Zuo, H.; Li, Y.; Liao, Y. Europium Ionic Liquid Grafted Covalent Organic Framework with Dual Luminescence Emissions as Sensitive and Selective Acetone Sensor. ACS Appl. Mater. Interfaces 2019, 11, 39201–39208. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, F.; Prabhakar, A.; Qin, X.; Forzani, E.S.; Tao, N. Colorimetric Sensor for Online Accurate Detection of Breath Acetone. ACS Sens. 2021, 6, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Chae, S.K.; Kim, J.-M. Colorimetric Sensors for Volatile Organic Compounds (VOCs) Based on Conjugated Polymer-Embedded Electrospun Fibers. J. Am. Chem. Soc. 2007, 129, 3038–3039. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Mo, W.; Tang, H.; Cheng, Z.; Chen, Y.; Zhang, S.; Ma, H.; Li, B.; Li, X. Dendrimer-Based, High-Luminescence Conjugated Microporous Polymer Films for Highly Sensitive and Selective Volatile Organic Compound Sensor Arrays. Adv. Funct. Mater. 2020, 30, 1910275. [Google Scholar] [CrossRef]
- Kau, N.; Jindal, G.; Kaur, R.; Rana, S. Progress in development of metal organic frameworks for electrochemical sensing of volatile organic compounds. Results Chem. 2022, 4, 100678. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal–organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Kang, X.; Deng, N.; Yan, Z.; Pan, Y.; Sun, W.; Zhang, Y. Resistive-type VOCs and pollution gases sensor based on SnO2: A review. Mater. Sci. Semicond. Process. 2022, 138, 106246. [Google Scholar] [CrossRef]
- Gautam, V.; Kumar, A.; Kumar, R.; Husain, M.; Jain, V.K.; Nagpal, S. Ultrafast trace-level detection of methyl nicotinate biomarker using TiO2/SiNWs nanocomposite-based sensing platform. J. Mater. Sci. Mater. Electron. 2022, 33, 3411–3423. [Google Scholar] [CrossRef]
- Li, J.; Xian, J.; Wang, W.; Cheng, K.; Zeng, M.; Zhang, A.; Wu, S.; Gao, X.; Lu, X.; Liu, J.-M. Ultrafast response and high-sensitivity acetone gas sensor based on porous hollow Ru-doped SnO2 nanotubes. Sens. Actuators B Chem. 2022, 352, 131061. [Google Scholar] [CrossRef]
- Qiu, J.; Yu, T.; Zhang, W.; Zhao, Z.; Zhang, Y.; Ye, G.; Zhao, Y.; Du, X.; Liu, X.; Yang, L.; et al. A Bioinspired, Durable, and Nondisposable Transparent Graphene Skin Electrode for Electrophysiological Signal Detection. ACS Mater. Lett. 2020, 2, 999–1007. [Google Scholar] [CrossRef]
- Polavarapu, L.; Liz-Marzán, L.M. Growth and galvanic replacement of silver nanocubes in organic media. Nanoscale 2013, 5, 4355–4361. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yi, L.; Wang, L.; Yao, J.; Militky, J.; Venkataramam, M.; Wiener, J.; Zhang, M. Preparation of core-sheath nanofibers with high latent heat by thermal cross-linking and coaxial electrospinning. Polymer 2021, 228, 123958. [Google Scholar] [CrossRef]
- Lei, S.; Quan, Z.; Qin, X.; Yu, J. Asymptotic decay of velocity of whipping jet in electrospinning. Polymer 2021, 217, 123456. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Leng, D.; Ma, F.; Zhang, Z.; Zhang, Y.; Wang, W.; Liang, Q.; Gao, J.; Lu, H. Nanoscale Pd catalysts decorated WO3–SnO2 heterojunction nanotubes for highly sensitive and selective acetone sensing. Sens. Actuators B Chem. 2020, 306, 127575. [Google Scholar] [CrossRef]
- Fan, Z.; Zhi, C.; Wu, L.; Zhang, P.; Feng, C.; Deng, L.; Yu, B.; Qian, L. UV/Ozone-Assisted Rapid Formation of High-Quality Tribological Self-Assembled Monolayer. Coatings 2019, 9, 762. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.; Roy, S.; Bhattacharya, G.; Fishlock, S.; Deshmukh, S.; Bhowmick, S.; McLaughlign, J.; Roy, S.S. Potentiometric ion-selective sensors based on UV-ozone irradiated laser-induced graphene electrode. Electrochim. Acta 2021, 387, 138341. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, X.; Guan, W.; Long, H.; Zhang, L.; Wang, H. Self-templated flower-like WO3-In2O3 hollow microspheres for conductometric acetone sensors. Sens. Actuators B 2022, 361, 131705. [Google Scholar] [CrossRef]
- Jin, S.; Wu, D.; Song, W.; Hao, H.; Gao, W.; Yan, S. Superior acetone sensor based on hetero-interface of SnSe2/SnO2 quasi core shell nanoparticles for previewing diabetes. J. Colloid Interface Sci. 2022, 621, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Americo, S.; Pargoletti, E.; Soave, R.; Cargnoni, F.; Trioni, M.I.; Chiarello, G.L.; Cerrato, G.; Cappelletti, G. Unveiling the acetone sensing mechanism by WO3 chemiresistors through a joint theory-experiment approach. Electrochim. Acta 2021, 371, 137611. [Google Scholar] [CrossRef]
- Alshareef, M.; Snari, R.M.; Alaysuy, O.; Aldawsari, A.M.; Abumelha, H.M.; Katouah, H.; El-Metwaly, N.M. Optical Detection of Acetone Using “Turn-Off” Fluorescent Rice Straw Based Cellulose Carbon Dots Imprinted onto Paper Dipstick for Diabetes Monitoring. ACS Omega 2022, 7, 16766–16777. [Google Scholar] [CrossRef]
- Cai, Z.; Park, S. Highly selective acetone sensor based on Co3O4-decorated porous TiO2 nanofibers. J. Alloys Compd. 2022, 919, 165875. [Google Scholar] [CrossRef]
- Jin, R.; Jiang, Y.; Zhao, L.; Wang, T.; Liu, X.; Liu, F.; Yan, X.; Sun, P.; Lu, G. High sensitivity and low detection limit of acetone sensor based on Ru-doped Co3O4 flower-like hollow microspheres. Sens. Actuators B 2022, 363, 131839. [Google Scholar] [CrossRef]







| Sample | Applied Voltage (kV) | Fiber Diameter (nm) |
|---|---|---|
| SnO2/Ag/PMMA | 10 | 78 ± 16 |
| 15 | 69 ± 38 | |
| 20 | 80 ±54 | |
| WO3/Ag/PMMA | 10 | 122 ± 32 |
| 15 | 75 ± 23 | |
| 20 | 72 ± 30 | |
| WO3/SnO2/Ag/PMMA | 10 | 132 ± 68 |
| 15 | 61 ± 22 | |
| 20 | 82 ± 26 |
| VOCs | Detection Limit (ppm) | △E | Responses Time (min) |
|---|---|---|---|
| Ethanol | 100 | −0.087 | 10 |
| p−Xylene | 80 | −0.061 | 20 |
| Toluene | 80 | −0.058 | 25 |
| Acetone | 20 | −0.081 | 10 |
| Materials | Sensor Type | Detection Limit | Detection Time | Operation Temp. (°C) | Ref. |
|---|---|---|---|---|---|
| WO3−In2O3 | conductometric | 0.1 ppm | real−time | 280 | [37] |
| SnSe2/SnO2 | conductometric | 0.354 ppm | real−time | 300 | [38] |
| γ−WO3 | chemiresistive | 0.2 ppm | 20 s | 300 | [39] |
| N−doped carbon dots | colorimetric | 0.5 mM | − | room temperature | [40] |
| Co3O4−TiO2 | conductometric | 0.1 ppm | 122 s | 250 | [41] |
| Ru−Co3O4 | conductometric | 50 ppb | real−time | 137.5 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-H.; Hsieh, T.-H.; Hsiao, K.-C.; Lin, T.-H.; Hsu, K.-H.; Wu, M.-C. Electrospun Fibrous Nanocomposite Sensing Materials for Monitoring Biomarkers in Exhaled Breath. Polymers 2023, 15, 1833. https://doi.org/10.3390/polym15081833
Chang Y-H, Hsieh T-H, Hsiao K-C, Lin T-H, Hsu K-H, Wu M-C. Electrospun Fibrous Nanocomposite Sensing Materials for Monitoring Biomarkers in Exhaled Breath. Polymers. 2023; 15(8):1833. https://doi.org/10.3390/polym15081833
Chicago/Turabian StyleChang, Yin-Hsuan, Ting-Hung Hsieh, Kai-Chi Hsiao, Ting-Han Lin, Kai-Hsiang Hsu, and Ming-Chung Wu. 2023. "Electrospun Fibrous Nanocomposite Sensing Materials for Monitoring Biomarkers in Exhaled Breath" Polymers 15, no. 8: 1833. https://doi.org/10.3390/polym15081833