Preparation and Characterization of New Bioplastics Based on Polybutylene Succinate (PBS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
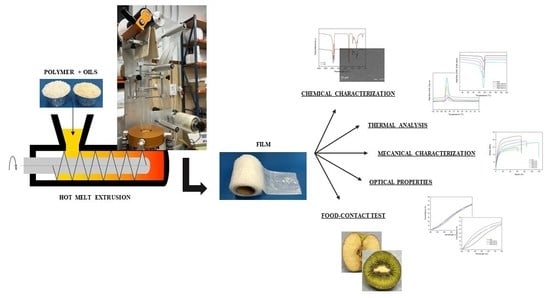
Polymer and Oils
2.2. Preparation
2.2.1. Extrusion
2.2.2. Film Manufacturing
2.3. Chemico-Physical and Mechanical Charaterization
2.3.1. Thermal Characterization
2.3.2. Chemical and Morphological Characterization
2.3.3. Mechanical Characterization
2.3.4. Optical Properties
2.3.5. Static Water Contact-Angle Measurements
2.3.6. Food-Contact Test
3. Results
3.1. Thermal Characterization
3.2. Chemical and Morphological Characterization
3.3. Mechanical Charaterization
3.4. Optical Properties
3.5. Water-Absorption Analysis
3.6. Food-Contact Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kummu, M.; De Moel, H.; Porkka, M.; Siebert, S.; Varis, O.; Ward, P.J. Lost food, wasted resources: Global food supply chain losses and their impacts on freshwater, cropland, and fertiliser use. Sci. Total Environ. 2012, 438, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Stenmarck, Â.; Jensen, C.; Quested, T.; Moates, G.; Buksti, M.; Cseh, B.; Juul, S.; Parry, A.; Politano, A.; Redlingshofer, B.; et al. Estimates of European Food Waste Levels; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016; p. 79. Available online: https://research.wur.nl/en/publications/estimates-of-european-food-waste-levels (accessed on 1 January 2023).
- Anastasopoulou, A.; Fortibuoni, T. Impact of plastic pollution on marine life in the Mediterranean Sea. In Plastics in the Aquatic Environment—Part I; Springer: Cham, Switzerland, 2019; pp. 135–196. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.S.F.K.S.; Sherwani, S.F.K. Nanocellulose reinforced thermoplastic starch (TPS), polylactic acid (PLA), and polybutylene succinate (PBS) for food packaging applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Fetting, C. The European Green Deal. In ESDN Report; ESDN Office: Vienna, Austria, 2020. [Google Scholar]
- Zamri, G.B.; Azizal, N.K.A.; Nakamura, S.; Okada, K.; Nordin, N.H.; Othman, N.; Akhir, F.N.M.D.; Kaida, N.; Hara, H. Delivery, impact and approach of household food waste reduction campaigns. J. Clean. Prod. 2020, 246, 118969. [Google Scholar] [CrossRef]
- Mangara, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Package Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A review on properties and application of bio-based poly (butylene succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Pennacchio, A.; Ventorino, V.; Cimini, D.; Pepe, O.; Schiraldi, C.; Inverso, M.; Faraco, V. Isolation of new cellulase and xylanase producing strains and application to lignocellulosic biomasses hydrolysis and succinic acid production. Bioresour. Technol. 2018, 259, 325–333. [Google Scholar] [CrossRef]
- Mohamad, N.; Mohamad Mazlan, M.; Tawakkal, I.S.M.A.; Talib, R.A.; Kian, L.K.; Jawaid, M. Characterization of active polybutylene succinate films filled essential oils for food packaging application. J. Polym. Environ. 2022, 30, 585–596. [Google Scholar] [CrossRef]
- Stoleru, E.; Brebu, M. Stabilization techniques of essential oils by incorporation into biodegradable polymeric materials for food packaging. Molecules 2021, 26, 6307. [Google Scholar] [CrossRef]
- Nikolic, M.S.; Djonlagic, J. Synthesis and characterization of biodegradable poly (butylene succinate-co-butylene adipate) s. Polym. Degrad. Stab. 2001, 74, 263–270. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B. Potential use of FTIR-ATR spectroscopic method for determination of virgin coconut oil and extra virgin olive oil in ternary mixture systems. Food Anal. Methods 2011, 4, 155–162. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Ramis-Ramos, G.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Authentication of extra virgin olive oils by Fourier-transform infrared spectroscopy. Food Chem. 2010, 118, 78–83. [Google Scholar] [CrossRef]
- Abderrahim, B.; Abderrahman, E.; Mohamed, A.; Fatima, T.; Abdesselam, T.; Krim, O. Kinetic thermal degradation of cellulose, polybutylene succinate and a green composite: Comparative study. World J. Environ. Eng. 2015, 3, 95–110. [Google Scholar] [CrossRef]
- Qiu, Z.; Komura, M.; Ikehara, T.; Nishi, T. DSC and TMDSC study of melting behaviour of poly (butylene succinate) and poly (ethylene succinate). Polymer 2003, 44, 7781–7785. [Google Scholar] [CrossRef]
- Fenni, S.E.; Wang, J.; Haddaoui, N.; Favis, B.D.; Müller, A.J.; Cavallo, D. Crystallization and self-nucleation of PLA, PBS and PCL in their immiscible binary and ternary blends. Thermochim. Acta 2019, 677, 117–130. [Google Scholar] [CrossRef]
- Blanco, I.; Poggetto, G.D.; Morrone, B.; Tranquillo, E.; Barrino, F.; Catauro, M. Fly Ash Filled Geopolymers: Preparation and Thermal Study. Macromol. Symp. 2020, 389, 1900052. [Google Scholar] [CrossRef]
- Peñas, M.I.; Pérez-Camargo, R.A.; Hernández, R.; Müller, A.J. A Review on Current Strategies for the Modulation of Thermomechanical, Barrier, and Biodegradation Properties of Poly (Butylene Succinate)(PBS) and Its Random Copolymers. Polymers 2022, 14, 1025. [Google Scholar] [CrossRef]
- De La Rosa-Ramírez, H.; Aldas, M.; Ferri, J.M.; López-Martínez, J.; Samper, M.D. Modification of poly (lactic acid) through the incorporation of gum rosin and gum rosin derivative: Mechanical performance and hydrophobicity. J. Appl. Polym. Sci. 2020, 137, 49346. [Google Scholar] [CrossRef]
- Rohman, A. Infrared spectroscopy for quantitative analysis and oil parameters of olive oil and virgin coconut oil: A review. Int. J. Food Prop. 2017, 20, 1447–1456. [Google Scholar] [CrossRef] [Green Version]
- Tranquillo, E.; Barrino, F.; Dal Poggetto, G.; Blanco, I. Sol–gel synthesis of silica-based materials with different percentages of PEG or PCL and high chlorogenic acid content. Materials 2019, 12, 155. [Google Scholar] [CrossRef] [Green Version]
- Catauro, M.; Barrino, F.; Pacifico, S.; Piccolella, S.; Lancellotti, I.; Leonelli, C. Synthesis of WEEE-based geopolymers and their cytotoxicity. Mater. Today Proc. 2021, 34, 121–124. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Dal Poggetto, G.; Milazzo, M.; Blanco, I.; Ciprioti, S.V. Structure, drug absorption, bioactive and antibacterial properties of sol-gel SiO2/ZrO2 materials. Ceram. Int. 2020, 46, 29459–29465. [Google Scholar] [CrossRef]
- Ferri, J.M.; Garcia-Garcia, D.; Sánchez-Nacher, L.; Fenollar, O.; Balart, R. The effect of maleinized linseed oil (MLO) on mechanical performance of poly (lactic acid)-thermoplastic starch (PLA-TPS) blends. Carbohyd. Polym. 2016, 147, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ferri, J.M.; Samper, M.D.; García-Sanoguera, D.; Reig, M.J.; Fenollar, O.; Balart, R. Plasticizing effect of biobased epoxidized fatty acid esters on mechanical and thermal properties of poly (lactic acid). J. Mater. Sci. 2016, 51, 5356–5366. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Jiménez-López, M.; Aldas, M.; López, J. Combined effect of linseed oil and gum rosin as natural additives for PVC. Ind. Crop. Prod. 2017, 99, 196–204. [Google Scholar] [CrossRef]
- Luzi, F.; Del Buono, D.; Orfei, B.; Moretti, C.; Bounaurio, R.; Torre, L.; Puglia, D. Lemna minor aqueous extract as a natural ingredient incorporated in poly (vinyl alcohol)-based films for active food packaging systems. Food Packag. Shelf Life 2022, 32, 100822. [Google Scholar] [CrossRef]










| Label | Composition | ||
|---|---|---|---|
| Polymer (wt%) | Olive Oil EVO (wt%) | Coconut Oil (wt%) | |
| PBS | 100 | --- | --- |
| PBS/CO 1 wt% | 99 | --- | 1 |
| PBS/CO 2 wt% | 98 | --- | 2 |
| PBS/CO 3 wt% | 97 | --- | 3 |
| PBS/EVO 1 wt% | 99 | 1 | --- |
| PBS/EVO 2 wt% | 98 | 2 | --- |
| PBS/EVO 3 wt% | 97 | 3 | --- |
| Samples | DSC (at a Stream Rate of 10 °C min−1) | ||||
|---|---|---|---|---|---|
| Tcc (°C) 1 | ΔHcc (Jg−1) 2 | Tm (°C) 3 | ΔHm (Jg−1) 4 | Xc (%) 5 | |
| PBS | 82.6 | 69.2 | 117.8 | 74.1 | 0.04 |
| PBS/CO 1 wt% | 87.2 | 66.9 | 116.8 | 74.9 | 0.07 |
| PBS/CO 2 wt% | 86.7 | 64.1 | 114.9 | 68.1 | 0.04 |
| PBS/CO 3 wt% | 86.7 | 66.3 | 114.4 | 71.6 | 0.05 |
| PBS/EVO 1 wt% | 86.9 | 70.8 | 116.7 | 76.2 | 0.05 |
| PBS/EVO 2 wt% | 87.9 | 63.3 | 116.3 | 68.8 | 0.05 |
| PBS/EVO 3 wt% | 86.8 | 66.7 | 115.2 | 72.1 | 0.05 |
| Peak (cm−1) | Assignment of Bonds | Mode of Vibration |
|---|---|---|
| 3006 | =C–H | Stretching |
| 2930 | =C–H (CH2) | Stretching (asymmetrical) |
| 2855 | =C–H (CH2) | Stretching (symmetrical) |
| 1747 | C=O | Stretching |
| 1466 | C–H (CH2) | Bending (scissoring) |
| 1415 | =C–H | Bending (rocking) |
| 1377 | C–H (CH2) | Bending (symmetrical) |
| 1161 | CH2 | Bending |
| 1113, 1095, 1028 | C–O | Stretching |
| 965 | CH=CH | Bending out of plane |
| 858 | =CH2 | Wagging |
| 722 | CH=CH | Bending out of plane |
| Sample | Tensile Strength (Mpa) | Tensile Modulus (Mpa) | Elongation at Break (%) |
|---|---|---|---|
| PBS | 22.5 ± 1.29 | 361.5 ± 1.91 | 71.1 ± 2.22 |
| PBS/CO 1 wt% | 15.0 ± 2.06 | 291.6 ± 10.5 | 59.2 ± 2.80 |
| PBS/CO 2 wt% | 14.8 ± 1.47 | 226.3 ± 9.44 | 62.4 ± 3.11 |
| PBS/CO 3 wt% | 13.8 ± 0.80 | 249.1 ± 9.99 | 71.6 ± 2.21 |
| PBS/EVO 1 wt% | 20.2 ± 1.71 | 336.4 ± 2.76 | 71.9 ± 2.95 |
| PBS/EVO 2 wt% | 19.0 ± 2.27 | 300.4 ± 4.18 | 85.9 ± 2.28 |
| PBS/EVO 3 wt% | 18.2 ± 1.42 | 244.8 ± 8.60 | 119.8 ± 3.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrino, F.; De La Rosa-Ramírez, H.; Schiraldi, C.; López-Martínez, J.; Samper, M.D. Preparation and Characterization of New Bioplastics Based on Polybutylene Succinate (PBS). Polymers 2023, 15, 1212. https://doi.org/10.3390/polym15051212
Barrino F, De La Rosa-Ramírez H, Schiraldi C, López-Martínez J, Samper MD. Preparation and Characterization of New Bioplastics Based on Polybutylene Succinate (PBS). Polymers. 2023; 15(5):1212. https://doi.org/10.3390/polym15051212
Chicago/Turabian StyleBarrino, Federico, Harrison De La Rosa-Ramírez, Chiara Schiraldi, Juan López-Martínez, and María Dolores Samper. 2023. "Preparation and Characterization of New Bioplastics Based on Polybutylene Succinate (PBS)" Polymers 15, no. 5: 1212. https://doi.org/10.3390/polym15051212