Effects of Europium Complex on Thermal and Photoluminescence Properties of Polyurethane-Europium Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Europium Complex
2.3. Preparation of Polyurethane–Europium Materials
2.4. Characterization
3. Results and Discussion
3.1. Structure of Polyurethane–Europium Materials
3.2. Dispersion of Europium Complex in Polyurethane
3.3. Transparency of Polyurethane–Europium Materials
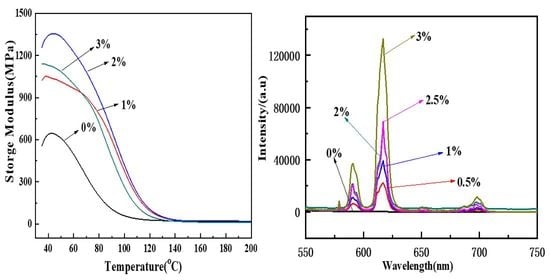
3.4. Thermal Stability of Polyurethane–Europium Materials
3.5. Luminescence of Polyurethane–Europium Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dasari, S.; Patra, A.K. Luminescent europium and terbium complexes of dipyridoquinoxaline and dipyridophenazine ligands as photosensitizing antennae: Structures and biological perspectives. Dalton Trans. 2015, 44, 19844–19855. [Google Scholar] [CrossRef]
- Baek, N.S.; Kim, Y.H.; Lee, D.H.; Seo, K.D.; Kim, H.K. Effect of coordination environment on the photophysical properties of luminescent europium(III) complexes. Bull. Korean Chem. Soc. 2009, 30, 1553–1558. [Google Scholar]
- Ung, P.; Clerc, M.; Huang, H.; Qiu, K.; Chao, H.; Seitz, M.; Boyd, B.; Graham, B.; Gasser, G. Extending the excitation wavelength of potential photosensitizers via appendage of a kinetically stable terbium(III) macrocyclic complex for applications in photodynamic therapy. Inorg. Chem. 2017, 56, 7960–7974. [Google Scholar] [CrossRef] [PubMed]
- Manseki, K.; Hasegawa, Y.; Wada, Y.; Yanagida, S. Photosensitized luminescence of thermostable polynuclear Eu(III) complexes. J. Lumin. 2005, 111, 183–189. [Google Scholar] [CrossRef]
- Zhu, M.M.; Zhang, Z.; Ren, N.; Wang, S.P.; Zhang, J.J. Rare earth complexes with 3,4-dimethylbenzoic acid and 2,2:6′,2″-terpyridine: Synthesis, crystal structures, luminescence and thermodynamic properties. Inorg. Chim. Acta 2019, 484, 311–318. [Google Scholar] [CrossRef]
- Chen, Y.M.; Li, L.; Zhang, Q.C.; Liu, S.S.; Tian, Z.F.; Ju, Z.H. Effects of calcium ions on crystal structure and luminescence properties of six rare earth metal complexes. J. Solid State Chem. 2020, 281, 121053. [Google Scholar] [CrossRef]
- Zhang, R.J.; Yang, K.Z.; Yu, A.C.; Zhao, X.S. Fluorescence lifetime and energy transfer of rare earth β-diketone complexes in organized molecular films. Thin Solid Films 2000, 363, 275–278. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.X.; Ao, B.Y.; Feng, S.Y.; Xin, X.D. Fluorescence enhancement of europium(III) perchlorate by benzoic acid on bis(benzylsulfinyl)methane complex and its binding characteristics with the bovine serum albumin (BSA). Spectrochim. Acta A 2014, 118, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.W.; Li, X.; Shi, X.Y.; Sun, X.J.; Sun, X.L. Enhanced luminescence of rare-earth Tb(III) by Tm(III) in bis(benzoylmethyl) sulfoxide complexes and intra-molecular energy transfer. J. Lumin. 2009, 129, 639–644. [Google Scholar] [CrossRef]
- Jiu, H.F.; Liu, G.D.; Zhang, Z.J.; Fu, Y.H.; Chen, J.C.; Fan, T.; Zhang, L.X. Fluorescence enhancement of Tb(III) complex with a new beta-diketone ligand by 1,10-phenanthroline. J. Rare Earths 2011, 29, 741–745. [Google Scholar] [CrossRef]
- Liu, C.L.; Zhang, R.L.; Lin, C.S.; Zhou, L.P.; Cai, L.X.; Kong, J.T.; Yang, S.Q.; Han, K.L.; Sun, Q.F. Intraligand charge transfer sensitization on self-assembled europium tetrahedral cage leads to dual-selective luminescent sensing toward anion and cation. J. Am. Chem. Soc. 2017, 139, 12474–12479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Zhu, Q.; Wang, X.J.; Li, X.D.; Sun, X.D.; Kim, B.N.; Li, J.G. Multi-color luminescent m-LaPO4:Ce/Tb monospheres of high efficiency via topotactic phase transition and elucidation of energy interaction. Inorg. Chem. 2019, 58, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Tang, P.S.; Hou, R.P.; Zhou, Y.F.; Chen, H.F. Effect of ligands on luminescent properties of rare earth europium complexes. Integr. Ferroelectr. 2021, 218, 35–42. [Google Scholar] [CrossRef]
- Barja, B.; Aramendia, P.; Baggio, R.; Garland, M.T.; Pena, O.; Perec, M. Europium(III) and terbium(III) trans-2-butenoates: Syntheses, crystal structures, and properties. Inorg. Chim. Acta 2003, 355, 183–190. [Google Scholar] [CrossRef]
- Sharma, G.; Narula, A.K. Synthesis of Eu(III) complexes with 2-aminopyridine and 1,10-phenanthroline: Structural, optical, thermal and morphological studies. Sens. Actuators B Chem. 2015, 215, 584–591. [Google Scholar] [CrossRef]
- Devi, R.; Bala, M.; Khatkar, S.P.; Taxak, V.B.; Boora, P. Investigations of luminescent behavior and intramolecular energy transfer mechanism of europium(III) complexes with fluorinated β-ketoester ligand. J. Fluor. Chem. 2016, 181, 36–44. [Google Scholar] [CrossRef]
- Singh-Wilmot, M.A.; Sinclair, R.A.; Kahwa, I.A.; Lough, A.J. Eu3+ substitutional defects and their effect on the luminescence spectral and decay dynamics of sal-type one dimensional rare earth coordination polymers and trinuclear complexes. J. Lumin. 2017, 182, 98–106. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Song, L.; Zhao, R.; Tan, M.C. High-performance and flexible shortwave infrared photodetectors using composites of rare earth-doped nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 2344–2351. [Google Scholar] [CrossRef]
- Wang, H.N.; Fang, L.; Zhang, Z.; Epaarachchi, J.; Li, L.Y.; Hu, X.; Lu, C.H.; Xu, Z.Z. Light-induced rare earth organic complex/shape-memory polymer composites with high strength and luminescence based on hydrogen bonding. Compos. Part A—Appl. Sci. Manuf. 2019, 125, 105525. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.W.; Zhang, Z.; Liu, Y.; Epaarachchi, J.; Fang, Z.G.; Fang, L.; Lu, C.H.; Xu, Z.Z. A Flexible multifunctional pan piezoelectric fiber with hydrophobicity, energy storage, and fluorescence. Polymers 2022, 14, 4573. [Google Scholar]
- Kesavan, A.V.; Kumar, M.P.; Rao, A.D.; Ramamurthy, P.C. Light management through up-conversion and scattering mechanism of rare earth nanoparticle in polymer photovoltaics. Opt. Mater. 2019, 94, 286–293. [Google Scholar] [CrossRef]
- Wang, D.M.; Yu, Y.L.; Ai, X.; Pan, H.W.; Zhang, H.L.; Dong, L.S. Polylactide/poly(butylene adipate-co-terephthalate)/rare earth complexes as biodegradable light conversion agricultural films. Polym. Adv. Technol. 2019, 30, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, A.; Pawar, A.; Hwang, T.R.; Lee, J.W.; Choi, M.W.; Kim, B.K.; Kang, Y.S. Wavelength conversion using rare earth doped oxides in polyolefin based nanocomposite films. Polym. Int. 2012, 61, 943–950. [Google Scholar] [CrossRef]
- Kamimura, M.; Kanayama, N.; Tokuzen, K.; Soga, K.; Nagasaki, Y. Near-infrared (1550 nm) in vivo bioimaging based on rare-earth doped ceramic nanophosphors modified with PEG-b-poly(4-vinylbenzylphosphonate). Nanoscale 2011, 3, 3705–3713. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Yu, Y.; Zhu, Y.N.; Ge, M.Q. Preparation and luminescence properties of rare-earth doped fiber with spectral blue-shift: SrAl2O4:Eu2+, Dy3+ phosphors/triarylsulfonium hexafluoroantimonate based on polypropylene substrate. J. Rare Earths 2017, 35, 530–535. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Shen, L.F.; Pun, E.Y.B.; Chen, B.J.; Lin, H. Multi-color fluorescence in rare earth acetylacetonate hydrate doped poly methyl methacrylate. Opt. Commun. 2014, 311, 111–116. [Google Scholar] [CrossRef]
- Rao, K.S.V.K.; Liu, H.G.; Lee, Y.I. Fluorescence spectroscopy of polymer systems doped with rare-earth metal ions and their complexes. Appl. Spectrosc. Rev. 2010, 45, 409–446. [Google Scholar]
- Wu, L.Y.; Chen, G.M.; Li, Z.B. Layered rare-earth hydroxide/polyacrylamide nanocomposite hydrogels with highly tunable photoluminescence. Small 2017, 13, 1604070. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hu, J.; Zhang, Q.J.; Bao, J.; Liu, W.H.; Gao, C.; Luo, Y.H. Local microstructure characterization of rare earth-doped PMMA with low-ion content by fluorescence EXAFS. J. Appl. Polym. Sci. 2006, 100, 1294–1298. [Google Scholar] [CrossRef]
- Gao, Z.H.; Wu, Y.W.; Xu, L.; Hao, H.X.; Wu, Q.Y.; Xie, H.D. Preparation and luminescent properties of Eu(III) organic complex and novel transparent ethylene-methyl acrylate copolymer (EMA) films doped with complexes. Opt. Mater. 2018, 85, 193–199. [Google Scholar] [CrossRef]
- Yu, L.P.; Zhang, X.; Wei, D.X.; Wu, B.; Jiang, X.R.; Chen, G.Q. Highly efficient fluorescent material based on rare-earth-modified polyhydroxyalkanoates. Biomacromolecules 2019, 20, 3233–3241. [Google Scholar] [CrossRef]
- Zhang, D.J.; Zhao, W.J.; Feng, Z.X.; Wu, Y.Z.; Huo, C.X.; He, L.; Lu, W.J. Preparation of polymer-rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties. e-Polymers 2019, 19, 15–22. [Google Scholar] [CrossRef]
- Wu, Y.W.; Hao, H.X.; Wu, Q.Y.; Gao, Z.H.; Xie, H.D. Preparation and luminescent properties of the novel polymer-rare earth complexes composed of poly(ethylene-co-acrylic acid) and europium ions. Opt. Mater. 2018, 80, 65–70. [Google Scholar] [CrossRef]
- Gao, B.J.; Zhang, L.Q.; Zhang, D.D. Synthesis and characterization of two novel Schiff base type macromolecular ligands and preliminary research on luminescent property of polymer-rare earth complexes. J. Polym. Res. 2018, 25, 41. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Chen, S.Y. Rare earth complexes using azobenzene-containing poly(aryl ether)s with different absorption wavelengths as macromolecular ligands: Synthesis, characterization, fluorescence properties and fabrication of fluorescent holographic micropatterns. RSC Adv. 2018, 8, 37348–37355. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.C.; Choi, N.J. Fabrication of functional polyurethane/rare earth nanocomposite membranes by electrospinning and its VOCs absorption capacity from air. Nanomaterials 2017, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Fang, L.; Men, J. Studies on preparation, structure and fluorescence emission of polymer-rare earth complexes composed of aryl carboxylic acid-functionalized polystyrene and Tb(III) ion. Polymer 2012, 53, 4709–4717. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, L.; Li, Y. Effect of substituent groups with two types on benzene ring on photoluminescence property of complexes of benzoic acid—Functionalized polystyrene with Eu(III) ion. J. Photochem. Photobiol. A Chem. 2016, 324, 23–32. [Google Scholar] [CrossRef]
- Yang, F.; Yuan, Y.; Sijbesma, R.P.; Chen, Y.L. Sensitized mechanoluminescence design toward mechanically induced intense red emission from transparent polymer films. Macromolecules 2020, 53, 905–912. [Google Scholar] [CrossRef]
- Chinya, I.; Sen, R.; Dhar, A. Synthesis and characterization of transparent erbium-ytterbium co-doped polymer nanocomposites for fabrication of polymer optical preform. Phys. Status Solidi A 2017, 214, 1600685. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; Kim, H.Y.; Khattab, T.A.; El-Naggar, M.E. Production of photoluminescent transparent poly(methyl methacrylate) for smart windows. Luminescence 2022, 37, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Yuen, U.E. Synthesis of thermoplastic polyurethane and its physical and shape memory properties. J. Appl. Polym. Sci. 2006, 102, 607–615. [Google Scholar] [CrossRef]
- Wang, L.D.; Tang, J.; Li, R.Z.; Zhang, T.; Tong, L.; Tang, J.; Xu, L. Synthesis and characterization of electro-optic polyurethane-imide and fabrication of optical waveguide device. High Perform. Polym. 2017, 29, 879–888. [Google Scholar] [CrossRef]
- Jang, J.Y.; Do, J.Y. Synthesis and evaluation of thermoplastic polyurethanes as thermo-optic waveguide materials. Polym. J. 2014, 46, 349–354. [Google Scholar] [CrossRef]
- Jiang, Y.; Da, Z.L.; Qiu, F.X.; Yang, D.Y.; Guan, Y.J.; Cao, G.R. Azo biphenyl polyurethane: Preparation, characterization and application for optical waveguide switch. Opt. Mater. 2018, 75, 858–868. [Google Scholar] [CrossRef]
- Kim, M.S.; Song, M.Y.; Jeon, B.; Lee, J.Y. Synthesis and electro-optic properties of novel polyurethane containing the nitrophenylazoresorcinoxy group. Polym. Int. 2012, 6, 1739–1744. [Google Scholar] [CrossRef]
- Qiu, F.X.; Zhang, W.; Liu, J.H.; Yang, D.Y. Optically active polyurethane containing asymmetric center: Preparation, characterization and thermo-optic properties. Polym.-Plast. Technol. Eng. 2010, 49, 1521–1526. [Google Scholar] [CrossRef]
- Moreno, J.; Arregui, F.J.; Matias, I.R. Fiber optic ammonia sensing employing novel thermoplastic polyurethane membranes. Sens. Actuators B Chem. 2005, 105, 419–424. [Google Scholar] [CrossRef]
- Robert, V.; Lemercier, G. A combined experimental and theoretical study of carboxylate coordination modes: A structural probe. J. Am. Chem. Soc. 2006, 128, 1183–1187. [Google Scholar] [CrossRef]
- Tsaryuk, V.I.; Zhuravlev, K.P. Peculiarities of the luminescence excitation of europium and terbium substituted benzoates. J. Lumin. 2021, 237, 118159. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Azab, H.A.; Kamel, R.M. Sensitive and selective fluorescent chemosensor for the detection of some organophosphorus pesticides using luminescent Eu(III) complex. J. Photochem. Photobiol. A 2016, 321, 33–40. [Google Scholar] [CrossRef]







| Content of Eu Complexes (wt%) | Fluorescence Decay Curve | Fluorescence Lifetime (ms) | R2 |
|---|---|---|---|
| 0.5 | I(t) = 351.260exp(−t/429562.543) + 940.652exp(−t/1164870) + 2.136 | 1.075 | 0.997 |
| 1 | I(t) = 1384.104exp(−t/769872.31) + 371.719exp(−t/1448860)+ 0.625 | 0.997 | 0.998 |
| 2 | I(t) = 1722.242exp(−t/818073.406) + 1722.24263exp(−t/818072.250) + 4.340 | 0.818 | 0.998 |
| 2.5 | I(t) = 1758.733exp(−t/784677.206) + 1758.733exp(−t/784676.809) + 3.644 | 0.786 | 0.998 |
| 3 | I(t) = 1660.884exp(−t/920974.651) + 1660.884exp(−t/920967.633) + 10.696 | 0.921 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Li, L.; Li, Y.; He, C.; Zhou, L.; Qu, X.; Fang, S. Effects of Europium Complex on Thermal and Photoluminescence Properties of Polyurethane-Europium Materials. Polymers 2023, 15, 1064. https://doi.org/10.3390/polym15051064
Gao L, Li L, Li Y, He C, Zhou L, Qu X, Fang S. Effects of Europium Complex on Thermal and Photoluminescence Properties of Polyurethane-Europium Materials. Polymers. 2023; 15(5):1064. https://doi.org/10.3390/polym15051064
Chicago/Turabian StyleGao, Lijun, Liuyang Li, Yunqiu Li, Congcong He, Liming Zhou, Xiongwei Qu, and Shaoming Fang. 2023. "Effects of Europium Complex on Thermal and Photoluminescence Properties of Polyurethane-Europium Materials" Polymers 15, no. 5: 1064. https://doi.org/10.3390/polym15051064