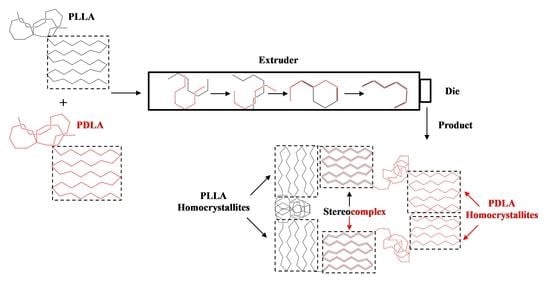
Scalable Continuous Manufacturing Process of Stereocomplex PLA by Twin-Screw Extrusion
Abstract
:1. Introduction
| Group | Method | fsc (%) | Tpm,sc (°C) | Xc (%) |
|---|---|---|---|---|
| Samsuri [41] | Solution casting with SC PLA particles | 60 | 220 | 23 |
| Arkanji [43] | Solution blending of PS-b-PDLA and P2VP-b-PLLA | >99 | 231 | 39 |
| Baimark [49] | Solution blending/precipitation of PLLA/PDLA (low MW) | >99 | 219 | 60 |
| Su [34] | Rheometer (190–220 °C) | >99 | 230 | 44 |
| Korber [38] | Co-rotating twin screw extrusion (180–240 °C) | 25 | 220 | 10 |
| Kara [48] | Counter-rotating twin screw extruder (220–235 °C) ⟶ single screw lab extruder (240 °C) | >99 | 220 | 55 |
| Alhaj | Co-rotating twin screw extruder (180–220 °C) | 95 | 240 | 58 |
2. Materials and Methods
2.1. Materials
2.2. Thermal Characteristics of Stereocomplex Formation
2.3. Twin-Screw Extrusion of Stereocomplex PLA
2.4. Injection Molding of Stereocomplex PLA
2.5. Characterization and Analysis
3. Results and Discussion
3.1. Thermal Characteristics of Stereocomplex Formation
3.2. Confirmation of Stereocomplex Formation by FTIR
3.3. Crystal Structure Characterization via WAXD
3.4. Thermal Characterization via DSC
3.5. Mechanical Properties of Stereocomplex PLA
3.6. Thermal Degradation via TGA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Mulla, M.Z.; Rahman, M.R.T.; Marcos, B.; Tiwari, B.; Pathania, S. Poly Lactic Acid (PLA) Nanocomposites: Effect of Inorganic Nanoparticles Reinforcement on Its Performance and Food Packaging Applications. Molecules 2021, 26, 1967. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Mangaraj, S.; Singh, R.P.; Das, S.K.; Naveenkumar, M.; Arora, S. Biopolymers as packaging material in food and allied industry. Int. J. Chem. Stud. 2018, 6, 2411–2418. [Google Scholar]
- Grijpma, D.W.; Pennings, A.J. (Co)polymers of L-lactide, 1. Synthesis, thermal properties and hydrolytic degradation. Macromol. Chem. Phys. 1994, 195, 1633–1647. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, B.; Bian, X.; Li, G.; Chen, Z.; Chen, X. Thermal Properties of Polylactides with Different Stereoisomers of Lactides Used as Comonomers. Macromolecules 2017, 50, 6064–6073. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) Stereocomplexes: Formation, Structure, Properties, Degradation, and Applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef]
- Ren, J.M.; Lawrence, J.; Knight, A.S.; Abdilla, A.; Zerdan, R.B.; Levi, A.E.; Oschmann, B.; Gutekunst, W.R.; Lee, S.-H.; Li, Y.; et al. Controlled Formation and Binding Selectivity of Discrete Oligo(methyl methacrylate) Stereocomplexes. J. Am. Chem. Soc. 2018, 140, 1945–1951. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Di Caprio, M.R.; Di Girolamo, R.; Ellis, W.C.; Coates, G.W. Stereocomplexed Poly(Limonene Carbonate): A Unique Example of the Cocrystallization of Amorphous Enantiomeric Polymers. Angew. Chemie Int. Ed. 2015, 54, 1215–1218. [Google Scholar] [CrossRef]
- Wu, G.; Jiang, S.; Lu, X.; Ren, W.; Yan, S. Stereoregular poly(cyclohexene carbonate)s: Unique crystallization behavior. Chinese J. Polym. Sci. 2012, 30, 487–492. [Google Scholar] [CrossRef]
- Jiang, Z.; Boyer, M.T.; Sen, A. Chiral and Steric Recognition in Optically Active, Isotactic, Alternating .alpha.-Olefin-Carbon Monoxide Copolymers. Effect on Physical Properties and Chemical Reactivity. J. Am. Chem. Soc. 1995, 117, 7037–7038. [Google Scholar] [CrossRef]
- Zhu, J.-B.; Watson, E.M.; Tang, J.; Chen, E.Y.-X. A synthetic polymer system with repeatable chemical recyclability. Science 2018, 360, 398–403. [Google Scholar] [CrossRef]
- Bertin, A. Emergence of Polymer Stereocomplexes for Biomedical Applications. Macromol. Chem. Phys. 2012, 213, 2329–2352. [Google Scholar] [CrossRef]
- Saravanan, M.; Domb, A.J. A contemporary review on—polymer stereocomplexes and its biomedical application. Eur. J. Nanomedicine 2013, 5, 81–96. [Google Scholar] [CrossRef]
- Tashiro, K.; Kouno, N.; Wang, H.; Tsuji, H. Crystal Structure of Poly(lactic acid) Stereocomplex: Random Packing Model of PDLA and PLLA Chains As Studied by X-ray Diffraction Analysis. Macromolecules 2017, 50, 8048–8065. [Google Scholar] [CrossRef]
- Slager, J.; Domb, A.J. Biopolymer stereocomplexes. Adv. Drug Deliv. Rev. 2003, 55, 549–583. [Google Scholar] [CrossRef]
- Slager, J.; Brizzolara, D.; Cantow, H.J.; Domb, A.J. Crystallization and stereocomplexation governed self-assembling of poly(lactide)-b-poly(ethylene glycol) to mesoscale structures. Polym. Adv. Technol. 2005, 16, 667–674. [Google Scholar] [CrossRef]
- Tsuji, H.; Yamasaki, M.; Arakawa, Y. Stereocomplex Formation between Enantiomeric Alternating Lactic Acid-Based Copolymers as a Versatile Method for the Preparation of High Performance Biobased Biodegradable Materials. ACS Appl. Polym. Mater. 2019, 1, 1476–1484. [Google Scholar] [CrossRef]
- Lamers, B.A.G.; van Genabeek, B.; Hennissen, J.; de Waal, B.F.M.; Palmans, A.R.A.; Meijer, E.W. Stereocomplexes of Discrete, Isotactic Lactic Acid Oligomers Conjugated with Oligodimethylsiloxanes. Macromolecules 2019, 52, 1200–1209. [Google Scholar] [CrossRef]
- Wan, Z.-Q.; Longo, J.M.; Liang, L.-X.; Chen, H.-Y.; Hou, G.-J.; Yang, S.; Zhang, W.-P.; Coates, G.W.; Lu, X.-B. Comprehensive Understanding of Polyester Stereocomplexation. J. Am. Chem. Soc. 2019, 141, 14780–14787. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Fortenberry, A.; Ren, J.; Qiang, Z. Recent Progress in Enhancing Poly(Lactic Acid) Stereocomplex Formation for Material Property Improvement. Front. Chem. 2020, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marin, P.; Tschan, M.J.-L.; Isnard, F.; Robert, C.; Haquette, P.; Trivelli, X.; Chamoreau, L.-M.; Guérineau, V.; del Rosal, I.; Maron, L.; et al. Polymerization of rac -Lactide Using Achiral Iron Complexes: Access to Thermally Stable Stereocomplexes. Angew. Chemie Int. Ed. 2019, 58, 12585–12589. [Google Scholar] [CrossRef] [PubMed]
- Brizzolara, D.; Cantow, H.-J.; Diederichs, K.; Keller, E.; Domb, A.J. Mechanism of the Stereocomplex Formation between Enantiomeric Poly(lactide)s. Macromolecules 1996, 29, 191–197. [Google Scholar] [CrossRef]
- Zhang, J.; Sato, H.; Tsuji, H.; Noda, I.; Ozaki, Y. Infrared Spectroscopic Study of CH 3 ···OC Interaction during Poly( l -lactide)/Poly( d -lactide) Stereocomplex Formation. Macromolecules 2005, 38, 1822–1828. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. In A New Era in Global Health; Springer Publishing Company: New York, NY, USA, 2018. [Google Scholar]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the Barrier Properties of PLA: A State-of-the-Art Review for Food Packaging Applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef]
- Varol, N. Advanced Thermal Analysis and Transport Properties of Stereocomplex Polylactide. Ph.D. Thesis, Normandie University, Le Havre, France, 2019. [Google Scholar]
- Jiang, Y.; Yan, C.; Wang, K.; Shi, D.; Liu, Z.; Yang, M. Super-Toughed PLA Blown Film with Enhanced Gas Barrier Property Available for Packaging and Agricultural Applications. Materials 2019, 12, 1663. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Zhou, J.; Fan, X.; Xie, H.; Zhang, H.; Zhang, G.; Shi, X. Influence of Polylactide (PLA) Stereocomplexation on the Microstructure of PLA/PBS Blends and the Cell Morphology of Their Microcellular Foams. Polymers 2020, 12, 2362. [Google Scholar] [CrossRef]
- Tsuji, H.; Tsuruno, T. Water Vapor Permeability of Poly(L-lactide)/Poly(D-lactide) Stereocomplexes. Macromol. Mater. Eng. 2010, 295, 709–715. [Google Scholar] [CrossRef]
- Gupta, A.; Mulchandani, N.; Shah, M.; Kumar, S.; Katiyar, V. Functionalized chitosan mediated stereocomplexation of poly(lactic acid): Influence on crystallization, oxygen permeability, wettability and biocompatibility behavior. Polymer 2018, 142, 196–208. [Google Scholar] [CrossRef]
- Jeong, J.; Ayyoob, M.; Kim, J.-H.; Nam, S.W.; Kim, Y.J. In situ formation of PLA-grafted alkoxysilanes for toughening a biodegradable PLA stereocomplex thin film. RSC Adv. 2019, 9, 21748–21759. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Feng, L.; Yu, D. Formation of Stereocomplex Crystal and Its Effect on the Morphology and Property of PDLA/PLLA Blends. Polymers 2020, 12, 2515. [Google Scholar] [CrossRef] [PubMed]
- Srithep, Y.; Pholharn, D.; Akkaprasa, T. Effect of molecular weight of poly(L-lactic acid) on the stereocomplex formation between enantiomeric poly(lactic acid)s blendings. IOP Conf. Ser. Mater. Sci. Eng. 2019, 526, 012024. [Google Scholar] [CrossRef]
- Tarkhanov, E.; Lehmann, A.; Menrath, A. Halfway to Technical Stereocomplex PLA Products—An Arduous Path to a Breakthrough. Macromol. Mater. Eng. 2020, 305, 1–6. [Google Scholar] [CrossRef]
- Scheuer, K.; Bandelli, D.; Helbing, C.; Weber, C.; Alex, J.; Max, J.B.; Hocken, A.; Stranik, O.; Seiler, L.; Gladigau, F.; et al. Self-Assembly of Copolyesters into Stereocomplex Crystallites Tunes the Properties of Polyester Nanoparticles. Macromolecules 2020, 53, 8340–8351. [Google Scholar] [CrossRef]
- Körber, S.; Moser, K.; Diemert, J. Development of High Temperature Resistant Stereocomplex PLA for Injection Moulding. Polymers 2022, 14, 384. [Google Scholar] [CrossRef]
- Xie, Q.; Han, L.; Shan, G.; Bao, Y.; Pan, P. Polymorphic Crystalline Structure and Crystal Morphology of Enantiomeric Poly(lactic acid) Blends Tailored by a Self-Assemblable Aryl Amide Nucleator. ACS Sustain. Chem. Eng. 2016, 4, 2680–2688. [Google Scholar] [CrossRef]
- Si, W.-J.; Zhang, H.; Li, Y.-D.; Huang, C.; Weng, Y.-X.; Zeng, J.-B. Highly toughened and heat resistant poly(l-lactide)/poly(ε-caprolactone) blends via engineering balance between kinetics and thermodynamics of phasic morphology with stereocomplex crystallite. Compos. Part B Eng. 2020, 197, 108155. [Google Scholar] [CrossRef]
- Samsuri, M.; Iswaldi, I.; Purnama, P. The Effect of Stereocomplex Polylactide Particles on the Stereocomplexation of High Molecular Weight Polylactide Blends. Polymers 2021, 13, 2018. [Google Scholar] [CrossRef]
- Qi, L.; Zhu, Q.; Cao, D.; Liu, T.; Zhu, K.R.; Chang, K.; Gao, Q. Preparation and Properties of Stereocomplex of Poly(lactic acid) and Its Amphiphilic Copolymers Containing Glucose Groups. Polymers 2020, 12, 760. [Google Scholar] [CrossRef]
- Arkanji, A.; Ladelta, V.; Ntetsikas, K.; Hadjichristidis, N. Synthesis and Thermal Analysis of Non-Covalent PS-b-SC-b-P2VP Triblock Terpolymers via Polylactide Stereocomplexation. Polymers 2022, 14, 2431. [Google Scholar] [CrossRef]
- Butron, A.; Llorente, O.; Fernandez, J.; Meaurio, E.; Sarasua, J.-R. Morphology and mechanical properties of poly(ethylene brassylate)/cellulose nanocrystal composites. Carbohydr. Polym. 2019, 221, 137–145. [Google Scholar] [CrossRef]
- Liang, L.; Huang, C.; Hao, N.; Ragauskas, A.J. Cross-linked poly(methyl vinyl ether-co-maleic acid)/poly(ethylene glycol)/nanocellulosics foams via directional freezing. Carbohydr. Polym. 2019, 213, 346–351. [Google Scholar] [CrossRef]
- Ren, Q.; Wu, M.; Weng, Z.; Zhu, X.; Li, W.; Huang, P.; Wang, L.; Zheng, W.; Ohshima, M. Promoted formation of stereocomplex in enantiomeric poly(lactic acid)s induced by cellulose nanofibers. Carbohydr. Polym. 2022, 276, 118800. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-R.; Niu, B.; Hua, W.-Q.; Li, Y.; Xu, L.; Wang, Y.; Ji, X.; Zhong, G.-J.; Li, Z.-M. Rapid preparation and continuous processing of polylactide stereocomplex crystallite below its melting point. Polym. Bull. 2019, 76, 3371–3385. [Google Scholar] [CrossRef]
- Kara, Y.; Molnár, K. Decomposition Behavior of Stereocomplex PLA Melt-Blown Fine Fiber Mats in Water and in Compost. J. Polym. Environ. 2022. [Google Scholar] [CrossRef] [PubMed]
- Baimark, Y.; Srihanam, P.; Srisuwan, Y.; Phromsopha, T. Enhancement in Crystallizability of Poly(L-Lactide) Using Stereocomplex-Polylactide Powder as a Nucleating Agent. Polymers 2022, 14, 4092. [Google Scholar] [CrossRef]
- Total Energies Corbion. "Product Data Sheet Luminy® L175," 06/0974 datasheet. 2017. [Revised 2019]. Available online: https://www.totalenergies-corbion.com/media/eushodia/pds-luminy-l175-190507.pdf (accessed on 6 January 2022).
- Total Energies Corbion. "Product Data Sheet Luminy® D120," 10/0966 datasheet. 2017. [Revised 2019]. Available online: https://www.totalenergies-corbion.com/media/0mxj0y1o/pds-luminy-d120-190507.pdf (accessed on 6 January 2022).
- D3418-21; Standard Test Method for Transition Temperatures and Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning Calometry. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- ISO 11357-3:2018; Plastics—Differential scanning calorimetry (DSC)—Part 3: Determination of temperature and enthalpy of melting and crystallization. ISO: Geneva, Switzerland, 2018.
- Sarasua, J.R.; Arraiza, A.L.; Balerdi, P.; Maiza, I. Crystallinity and mechanical properties of optically pure polylactides and their blends. Polym. Eng. Sci. 2005, 45, 745–753. [Google Scholar] [CrossRef]
- Biela, T.; Studies, M. Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-36199-9. [Google Scholar]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Tsuji, H.; Horii, F.; Nakagawa, M.; Ikada, Y.; Odani, H.; Kitamaru, R. Stereocomplex formation between enantiomeric poly(lactic acid)s. 7. Phase structure of the stereocomplex crystallized from a dilute acetonitrile solution as studied by high-resolution solid-state carbon-13 NMR spectroscopy. Macromolecules 1992, 25, 4114–4118. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acids). 9. Stereocomplexation from the melt. Macromolecules 1993, 26, 6918–6926. [Google Scholar] [CrossRef]
- Bouapao, L.; Tsuji, H. Stereocomplex Crystallization and Spherulite Growth of Low Molecular Weight Poly(L-lactide) and Poly(D-lactide) from the Melt. Macromol. Chem. Phys. 2009, 210, 993–1002. [Google Scholar] [CrossRef]
- Tsuji, H.; Fukui, I. Enhanced thermal stability of poly(lactide)s in the melt by enantiomeric polymer blending. Polymer 2003, 44, 2891–2896. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid Z. Und Z. Für Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- Hyvärinen, M.; Jabeen, R.; Kärki, T. The Modelling of Extrusion Processes for Polymers—A Review. Polymers 2020, 12, 1306. [Google Scholar] [CrossRef]
- Martin, C. Twin Screw Extruders as Continuous Mixers for Thermal Processing: A Technical and Historical Perspective. AAPS PharmSciTech 2016, 17, 3–19. [Google Scholar] [CrossRef]
- Sakai, T. Screw extrusion technology—past, present and future. Polimery 2013, 58, 847–857. [Google Scholar] [CrossRef]
- Bao, R.-Y.; Yang, W.; Jiang, W.-R.; Liu, Z.-Y.; Xie, B.-H.; Yang, M.-B.; Fu, Q. Stereocomplex formation of high-molecular-weight polylactide: A low temperature approach. Polymer 2012, 53, 5449–5454. [Google Scholar] [CrossRef]
- Cui, J.; Yang, S.-G.; Zhang, Q.; Liu, F.; Ungar, G. Poisoning by Purity: What Stops Stereocomplex Crystallization in Polylactide Racemate? Macromolecules 2023, 56, 989–998. [Google Scholar] [CrossRef]
- Zhang, J.; Sato, H.; Tsuji, H.; Noda, I.; Ozaki, Y. Differences in the CH3⋯O=C interactions among poly(L-lactide), poly(L-lactide)/poly(D-lactide) stereocomplex, and poly(3-hydroxybutyrate) studied by infrared spectroscopy. J. Mol. Struct. 2005, 735, 249–257. [Google Scholar] [CrossRef]
- Alemán, C.; Lotz, B.; Puiggali, J. Crystal Structure of the α-Form of Poly(L-lactide). Macromolecules 2001, 34, 4795–4801. [Google Scholar] [CrossRef]
- Hoogsteen, W.; Postema, A.R.; Pennings, A.J.; Ten Brinke, G.; Zugenmaier, P. Crystal structure, conformation and morphology of solution-spun poly(L-lactide) fibers. Macromolecules 1990, 23, 634–642. [Google Scholar] [CrossRef]
- De Santis, P.; Kovacs, A.J. Molecular conformation of poly(S-lactic acid). Biopolymers 1968, 6, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Asahi, T.; Ichiki, M.; Oikawa, A.; Suzuki, H.; Watanabe, T.; Fukada, E.; Shikinami, Y. Structural and optical properties of poly lactic acids. J. Appl. Phys. 1995, 77, 2957–2973. [Google Scholar] [CrossRef]
- Okihara, T.; Tsuji, M.; Kawaguchi, A.; Katayama, K.-I.; Tsuji, H.; Hyon, S.-H.; Ikada, Y. Crystal structure of stereocomplex of poly(L-lactide) and poly(D-lactide). J. Macromol. Sci. Part B 1991, 30, 119–140. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Investigation of Phase Transitional Behavior of Poly(L-lactide)/Poly(D-lactide) Blend Used to Prepare the Highly-Oriented Stereocomplex. Macromolecules 2007, 40, 1049–1054. [Google Scholar] [CrossRef]
- Srithep, Y.; Pholharn, D.; Turng, L.; Veang-in, O. Injection molding and characterization of polylactide stereocomplex. Polym. Degrad. Stab. 2015, 120, 290–299. [Google Scholar] [CrossRef]
- Aliotta, L.; Sciara, L.M.; Cinelli, P.; Canesi, I.; Lazzeri, A. Improvement of the PLA Crystallinity and Heat Distortion Temperature Optimizing the Content of Nucleating Agents and the Injection Molding Cycle Time. Polymers 2022, 14, 977. [Google Scholar] [CrossRef]
- Beauson, J.; Schillani, G.; Van der Schueren, L.; Goutianos, S. The effect of processing conditions and polymer crystallinity on the mechanical properties of unidirectional self-reinforced PLA composites. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106668. [Google Scholar] [CrossRef]
- Srithep, Y.; Pholharn, D.; Morris, J. Injection-molded poly(L-lactic acid)/poly(D-lactic acid) blends: Thermal and mechanical properties. In Proceedings of the AIP Conference Proceedings; American Institute of Physics: Melville, NY, USA, 2019; Volume 2065. [Google Scholar] [CrossRef]
- Li, J.; Ye, W.; Fan, Z.; Lu, Z. Stereocomplex poly(lactic acid) vascular stents by 3D-printing with long chain branching structures: Toward desirable crystallization properties and mechanical performance. Polym. Adv. Technol. 2021, 32, 97–110. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, D.; Zhou, Z.; Wu, Y.; Li, H.; Zhao, C.; Li, Y. Biaxial Stretching of Polymer Nanocomposites: A Mini-Review. Front. Mater. 2021, 8, 725422. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, W.; Liu, S.; Ji, H.; Chen, X.; Zhu, H.; Zhao, H.; Ma, Y.; Xie, L. The Formation of a Highly Oriented Structure and Improvement of Properties in PP/PA6 Polymer Blends during Extrusion-Stretching. Polymers 2020, 12, 878. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Wang, G.; Zhang, A.; Li, S.; Zhao, J.; Zhao, G.; Park, C.B. Ultra-ductile and strong in-situ fibrillated PLA/PTFE nanocomposites with outstanding heat resistance derived by CO2 treatment. Compos. Part A Appl. Sci. Manuf. 2022, 155, 106849. [Google Scholar] [CrossRef]













| Sample | o.p. (%) | σult (MPa) | E (GPa) | Tg (oC) | Tpm (oC) | Xc (%) | Mn (kDa) | Mw (kDa) | PDI |
|---|---|---|---|---|---|---|---|---|---|
| L175 | 99.68 | 50 | 3.5 | 63.13 | 175.10 | 47.52 | 103 | 172 | 1.67 |
| D120 | 99.81 | 50 | 3.5 | 62.21 | 177.52 | 51.40 | 92 | 150 | 1.64 |
| Collection Time (min) | fsc (%) | Xc (%) | Tpm,sc (°C) | Tpm,hc (°C) | Tg (°C) |
|---|---|---|---|---|---|
| 5 | 85 | 64 | 241 | 178 | 63 |
| 10 | 92 | 61 | 240 | 175 | 64 |
| 20 | 93 | 62 | 240 | 175 | 65 |
| 40 | 95 | 56 | 240 | 175 | 61 |
| Name | σbreak (MPa) | εbreak (%) | σy (MPa) | σult (MPa) | Eelastic (GPa) | εp (%) | UT (J/m3) | UR (MPa) |
|---|---|---|---|---|---|---|---|---|
| PLLA | 36.94 ± 1.5 | 9.62 ± 0.6 | 36.73 ± 1.3 | 48.67 ± 2.3 | 1.52 ± 0.07 | 7.18 ± 0.10 | 3460.17 ± 50.7 | 0.44 ± 0.02 |
| SC PLA | 96.29 ± 2.6 | 9.98 ± 1.2 | 70.87 ± 2.5 | 106.34 ± 3.1 | 2.93 ± 0.1 | 6.69 ± 0.08 | 8107.45 ± 65.3 | 0.86 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhaj, M.; Narayan, R. Scalable Continuous Manufacturing Process of Stereocomplex PLA by Twin-Screw Extrusion. Polymers 2023, 15, 922. https://doi.org/10.3390/polym15040922
Alhaj M, Narayan R. Scalable Continuous Manufacturing Process of Stereocomplex PLA by Twin-Screw Extrusion. Polymers. 2023; 15(4):922. https://doi.org/10.3390/polym15040922
Chicago/Turabian StyleAlhaj, Mohammed, and Ramani Narayan. 2023. "Scalable Continuous Manufacturing Process of Stereocomplex PLA by Twin-Screw Extrusion" Polymers 15, no. 4: 922. https://doi.org/10.3390/polym15040922