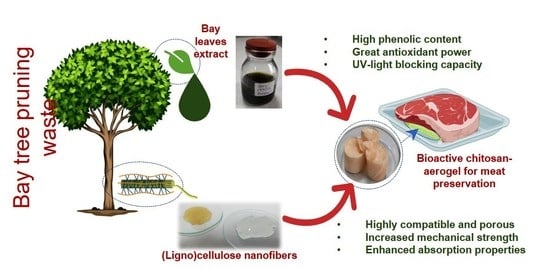
Bioactive Absorbent Chitosan Aerogels Reinforced with Bay Tree Pruning Waste Nanocellulose with Antioxidant Properties for Burger Meat Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production and Characterization of Bay Leaf Extract
2.3. BTPW Cellulose Pulp Production
2.4. BTPW-LCMNF and CMNF Production
2.5. BTPW Cellulose Fractions Characterization
2.5.1. Characterization of BTPW Cellulose Pulps
2.5.2. Characterization of LCMNF and CMNF
2.6. Preparation of (Bioactive) Chitosan Aerogels
2.7. Characterization of BTPW Micro/Nanofiber-Reinforced CH Aerogels
2.8. Characterization of Bioactive Aerogels
2.8.1. Radical Scavenging Activity by DPPH Assay
2.8.2. Evaluation of the Effect of Bioactive Aerogels on Meat Preservation
2.9. Statistics
3. Results and Discussion
3.1. Characterization of Bay Tree Pruning Waste Cellulose Fractions
3.2. Feasibility of BTPW-LCMNF and CMNF as an Enhancement for Chitosan Aerogels
3.3. Effect of Bay Leaf Extract on the Properties of Bioactive Aerogels
3.4. Evaluation of the Bioactive Capacity of BTPW-CH Aerogels for Meat Preservation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontes-Candia, C.; Erboz, E.; Martínez-Abad, A.; López-Rubio, A.; Martínez-Sanz, M. Superabsorbent Food Packaging Bioactive Cellulose-Based Aerogels from Arundo Donax Waste Biomass. Food Hydrocoll. 2019, 96, 151–160. [Google Scholar] [CrossRef]
- Otoni, C.G.; Espitia, P.J.P.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in Antimicrobial Food Packaging Systems: Emitting Sachets and Absorbent Pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Benito-González, I.; López-Rubio, A.; Galarza-Jiménez, P.; Martínez-Sanz, M. Multifunctional Cellulosic Aerogels from Posidonia Oceanica Waste Biomass with Antioxidant Properties for Meat Preservation. Int. J. Biol. Macromol. 2021, 185, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Hayden, M.; Qiao, M.; Huang, T.-S.; Ren, X.; Weese, J. Absorbent Pads Containing N-Halamine Compound for Potential Antimicrobial Use for Chicken Breast and Ground Chicken. J. Agric. Food Chem. 2018, 66, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, K.K.; Singh, S.; Ajji, A. Moisture Absorbers for Food Packaging Applications. Environ. Chem. Lett. 2019, 17, 609–628. [Google Scholar] [CrossRef]
- Kumar, S.; Basumatary, I.B.; Mukherjee, A.; Dutta, J. An Overview of Natural Biopolymers in Food Packaging. In Biopolymer-Based Food Packaging; Wiley: Hoboken, NJ, USA, 2022; pp. 1–28. [Google Scholar]
- Grujić, R.; Vujadinović, D.; Savanović, D. Biopolymers as Food Packaging Materials. In Advances in Applications of Industrial Biomaterials; Springer International Publishing: Cham, Switzerland, 2017; pp. 139–160. [Google Scholar]
- Zhang, W.; Jiang, H.; Rhim, J.-W.; Cao, J.; Jiang, W. Tea Polyphenols (TP): A Promising Natural Additive for the Manufacture of Multifunctional Active Food Packaging Films. Crit. Rev. Food Sci. Nutr. 2023, 63, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial Polyphenols as Potential Natural Food Preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef]
- Silva, F.; Domingues, F.C.; Nerín, C. Control Microbial Growth on Fresh Chicken Meat Using Pinosylvin Inclusion Complexes Based Packaging Absorbent Pads. LWT 2018, 89, 148–154. [Google Scholar] [CrossRef]
- Benito-González, I.; Jaén-Cano, C.M.; López-Rubio, A.; Martínez-Abad, A.; Martínez-Sanz, M. Valorisation of Vine Shoots for the Development of Cellulose-Based Biocomposite Films with Improved Performance and Bioactivity. Int. J. Biol. Macromol. 2020, 165, 1540–1551. [Google Scholar] [CrossRef]
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(Ligno)Nanocellulose Biocomposite Films. Effect of Residual Lignin Content on Structural, Mechanical, Barrier and Antioxidant Properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef]
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-Based Films Enriched with Nanocellulose-Stabilized Pickering Emulsions Containing Different Essential Oils for Possible Applications in Food Packaging. Food Packag. Shelf Life 2021, 27, 100615. [Google Scholar] [CrossRef]
- Mugwagwa, L.R.; Chimphango, A.F.A. Enhancing the Functional Properties of Acetylated Hemicellulose Films for Active Food Packaging Using Acetylated Nanocellulose Reinforcement and Polycaprolactone Coating. Food Packag. Shelf Life 2020, 24, 100481. [Google Scholar] [CrossRef]
- Rincón, E.; Espinosa, E.; García-Domínguez, M.T.; Balu, A.M.; Vilaplana, F.; Serrano, L.; Jiménez-Quero, A. Bioactive Pectic Polysaccharides from Bay Tree Pruning Waste: Sequential Subcritical Water Extraction and Application in Active Food Packaging. Carbohydr. Polym. 2021, 272, 118477. [Google Scholar] [CrossRef]
- Yang, W.; Weng, Y.; Puglia, D.; Qi, G.; Dong, W.; Kenny, J.M.; Ma, P. Poly(Lactic Acid)/Lignin Films with Enhanced Toughness and Anti-Oxidation Performance for Active Food Packaging. Int. J. Biol. Macromol. 2020, 144, 102–110. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Tabibiazar, M.; Ghorbani, M.; Jahanban-Esfahlan, A. Fabrication and Characterization of Novel Antibacterial Chitosan/Dialdehyde Guar Gum Hydrogels Containing Pomegranate Peel Extract for Active Food Packaging Application. Int. J. Biol. Macromol. 2021, 187, 179–188. [Google Scholar] [CrossRef]
- Fonseca, L.M.; da Silva, F.T.; Bruni, G.P.; Borges, C.D.; da Rosa Zavareze, E.; Dias, A.R.G. Aerogels Based on Corn Starch as Carriers for Pinhão Coat Extract (Araucaria Angustifolia) Rich in Phenolic Compounds for Active Packaging. Int. J. Biol. Macromol. 2021, 169, 362–370. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.P.; Bruni, G.P.; Fonseca, L.M.; da Silva, F.T.; da Rocha, J.C.; da Rosa Zavareze, E. Characterization of Aerogels as Bioactive Delivery Vehicles Produced through the Valorization of Yerba-Mate (Illex Paraguariensis). Food Hydrocoll. 2020, 107, 105931. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Dhua, S.; Gupta, A.K.; Mishra, P. Aerogel: Functional Emerging Material for Potential Application in Food: A Review. Food Bioprocess Technol. 2022, 15, 2396–2421. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Guo, Z.; Li, F.; Zhang, H.; Bai, F.; Wang, L. Chitosan-Based Bifunctional Composite Aerogel Combining Absorption and Phototherapy for Bacteria Elimination. Carbohydr. Polym. 2020, 247, 116739. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, P.; Song, X.; Liao, B.; Yan, K.; Zhang, J.-J. Facile Fabrication of Anisotropic Chitosan Aerogel with Hydrophobicity and Thermal Superinsulation for Advanced Thermal Management. ACS Sustain. Chem. Eng. 2021, 9, 9348–9357. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, S.; Han, F.; Li, M.; Wang, N.; Liu, L. Anisotropic Cellulose Nanofiber/Chitosan Aerogel with Thermal Management and Oil Absorption Properties. Carbohydr. Polym. 2021, 264, 118033. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Tarrés, Q.; Theng, D.; Delgado-Aguilar, M.; Rodríguez, A.; Mutjé, P. Effect of Enzymatic Treatment (Endo-Glucanases) of Fiber and Mechanical Lignocellulose Nanofibers Addition on Physical and Mechanical Properties of Binderless High-Density Fiberboards Made from Wheat Straw. J. Build. Eng. 2021, 44, 103392. [Google Scholar] [CrossRef]
- Peltola, H.; Immonen, K.; Johansson, L.; Virkajärvi, J.; Sandquist, D. Influence of Pulp Bleaching and Compatibilizer Selection on Performance of Pulp Fiber Reinforced PLA Biocomposites. J. Appl. Polym. Sci. 2019, 136, 47955. [Google Scholar] [CrossRef]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive Elucidation of the Effect of Residual Lignin on the Physical, Barrier, Mechanical and Surface Properties of Nanocellulose Films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef]
- Abe, K.; Nakatsubo, F.; Yano, H. High-Strength Nanocomposite Based on Fibrillated Chemi-Thermomechanical Pulp. Compos. Sci. Technol. 2009, 69, 2434–2437. [Google Scholar] [CrossRef]
- Rincón, E.; Balu, A.M.; Luque, R.; Serrano, L. Mechanochemical Extraction of Antioxidant Phenolic Compounds from Mediterranean and Medicinal Laurus Nobilis: A Comparative Study with Other Traditional and Green Novel Techniques. Ind. Crops Prod. 2019, 141, 111805. [Google Scholar] [CrossRef]
- Rincón, E.; Zuliani, A.; Jiménez-Quero, A.; Vilaplana, F.; Luque, R.; Serrano, L.; Balu, A.M. Combined Extraction/Purification-Catalytic Microwave-Assisted Conversion of Laurus Nobilis L. Pruning Waste Polysaccharides into Methyl Levulinate. ACS Sustain. Chem. Eng. 2020, 8, 11016–11023. [Google Scholar] [CrossRef]
- Rincón, E.; Serrano, L.; Balu, A.M.; Aguilar, J.J.; Luque, R.; García, A. Effect of Bay Leaves Essential Oil Concentration on the Properties of Biodegradable Carboxymethyl Cellulose-Based Edible Films. Materials 2019, 12, 2356. [Google Scholar] [CrossRef]
- Technical Association of the Pulp and Paper Industry (TAPPI) Technical Association of the Pulp and Paper Industry (TAPPI) Standards: Regulation and Style Guidelines. Available online: https://www.tappi.org/globalassets/documents/standards/tm_guidelines_complete.pdf2018 (accessed on 9 January 2023).
- Espinosa, E.; Sánchez, R.; Otero, R.; Domínguez-Robles, J.; Rodríguez, A. A Comparative Study of the Suitability of Different Cereal Straws for Lignocellulose Nanofibers Isolation. Int. J. Biol. Macromol. 2017, 103, 990–999. [Google Scholar] [CrossRef]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated Cellulose from TEMPO-Oxidized Eucalyptus Fibres: Effect of the Carboxyl Content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Espinosa, E.; Domínguez-Robles, J.; Sánchez, R.; Tarrés, Q.; Rodríguez, A. The Effect of Pre-Treatment on the Production of Lignocellulosic Nanofibers and Their Application as a Reinforcing Agent in Paper. Cellulose 2017, 24, 2605–2618. [Google Scholar] [CrossRef]
- Marx-Figini, M. The Acid-Catalyzed Degradation of Cellulose Linters in Distinct Ranges of Degree of Polymerization. J. Appl. Polym. Sci. 1987, 33, 2097–2105. [Google Scholar] [CrossRef]
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between Length and Degree of Polymerization of TEMPO-Oxidized Cellulose Nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef]
- Geng, H. A Facile Approach to Light Weight, High Porosity Cellulose Aerogels. Int. J. Biol. Macromol. 2018, 118, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Tang, J.; Faustman, C.; Hoagland, T.A. Krzywicki Revisited: Equations for Spectrophotometric Determination of Myoglobin Redox Forms in Aqueous Meat Extracts. J. Food Sci. 2006, 69, C717–C720. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Espinosa, E.; Bascón-Villegas, I.; Pérez-Rodríguez, F.; Carrasco, E.; Rodríguez, A. Production of Cellulose Nanofibers from Olive Tree Harvest—A Residue with Wide Applications. Agronomy 2020, 10, 696. [Google Scholar] [CrossRef]
- Bascón-Villegas, I.; Espinosa, E.; Sánchez, R.; Tarrés, Q.; Pérez-Rodríguez, F.; Rodríguez, A. Horticultural Plant Residues as New Source for Lignocellulose Nanofibers Isolation: Application on the Recycling Paperboard Process. Molecules 2020, 25, 3275. [Google Scholar] [CrossRef]
- Serra-Parareda, F.; Tarrés, Q.; Mutjé, P.; Balea, A.; Campano, C.; Sánchez-Salvador, J.L.; Negro, C.; Delgado-Aguilar, M. Correlation between Rheological Measurements and Morphological Features of Lignocellulosic Micro/Nanofibers from Different Softwood Sources. Int. J. Biol. Macromol. 2021, 187, 789–799. [Google Scholar] [CrossRef]
- Morcillo-Martín, R.; Espinosa, E.; Rabasco-Vílchez, L.; Sanchez, L.M.; de Haro, J.; Rodríguez, A. Cellulose Nanofiber-Based Aerogels from Wheat Straw: Influence of Surface Load and Lignin Content on Their Properties and Dye Removal Capacity. Biomolecules 2022, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Ehman, N.V.; Felissia, F.E.; Tarrés, Q.; Vallejos, M.E.; Delgado-Aguilar, M.; Mutjé, P.; Area, M.C. Effect of Nanofiber Addition on the Physical-Mechanical Properties of Chemimechanical Pulp Handsheets for Packaging. Cellulose 2020, 27, 10811–10823. [Google Scholar] [CrossRef]
- Chen, Y.; Geng, B.; Ru, J.; Tong, C.; Liu, H.; Chen, J. Comparative Characteristics of TEMPO-Oxidized Cellulose Nanofibers and Resulting Nanopapers from Bamboo, Softwood, and Hardwood Pulps. Cellulose 2017, 24, 4831–4844. [Google Scholar] [CrossRef]
- French, A.D. Idealized Powder Diffraction Patterns for Cellulose Polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Rizal, S.; Yahya, E.B.; Abdul Khalil, H.P.S.; Abdullah, C.K.; Marwan, M.; Ikramullah, I.; Muksin, U. Preparation and Characterization of Nanocellulose/Chitosan Aerogel Scaffolds Using Chemical-Free Approach. Gels 2021, 7, 246. [Google Scholar] [CrossRef]
- Meng, G.; Peng, H.; Wu, J.; Wang, Y.; Wang, H.; Liu, Z.; Guo, X. Fabrication of Superhydrophobic Cellulose/Chitosan Composite Aerogel for Oil/Water Separation. Fibers Polym. 2017, 18, 706–712. [Google Scholar] [CrossRef]
- Gao, C.; Wang, X.-L.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R. Synergistic Preparation of Modified Alginate Aerogel with Melamine/Chitosan for Efficiently Selective Adsorption of Lead Ions. Carbohydr. Polym. 2021, 256, 117564. [Google Scholar] [CrossRef]
- Takeshita, S.; Yoda, S. Chitosan Aerogels: Transparent, Flexible Thermal Insulators. Chem. Mater. 2015, 27, 7569–7572. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, M.; Li, M.; Liu, L.; Liu, L.; Yu, J. Cellulose Nanofibril (CNF) Based Aerogels Prepared by a Facile Process and the Investigation of Thermal Insulation Performance. Cellulose 2020, 27, 6217–6233. [Google Scholar] [CrossRef]
- Shang, K.; Yang, J.-C.; Cao, Z.-J.; Liao, W.; Wang, Y.-Z.; Schiraldi, D.A. Novel Polymer Aerogel toward High Dimensional Stability, Mechanical Property, and Fire Safety. ACS Appl. Mater. Interfaces 2017, 9, 22985–22993. [Google Scholar] [CrossRef]
- Luo, X.; Shen, J.; Ma, Y.; Liu, L.; Meng, R.; Yao, J. Robust, Sustainable Cellulose Composite Aerogels with Outstanding Flame Retardancy and Thermal Insulation. Carbohydr. Polym. 2020, 230, 115623. [Google Scholar] [CrossRef]
- Mueller, S.; Sapkota, J.; Nicharat, A.; Zimmermann, T.; Tingaut, P.; Weder, C.; Foster, E.J. Influence of the Nanofiber Dimensions on the Properties of Nanocellulose/Poly(Vinyl Alcohol) Aerogels. J. Appl. Polym. Sci. 2015, 132, 41740. [Google Scholar] [CrossRef]
- Ago, M.; Ferrer, A.; Rojas, O.J. Starch-Based Biofoams Reinforced with Lignocellulose Nanofibrils from Residual Palm Empty Fruit Bunches: Water Sorption and Mechanical Strength. ACS Sustain. Chem. Eng. 2016, 4, 5546–5552. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Development and Characterization of Carrageenan/Grapefruit Seed Extract Composite Films for Active Packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-Mechanical Properties of Chitosan Films with Carvacrol and Grape Seed Extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef]
- Benito-González, I.; López-Rubio, A.; Gómez-Mascaraque, L.G.; Martínez-Sanz, M. PLA Coating Improves the Performance of Renewable Adsorbent Pads Based on Cellulosic Aerogels from Aquatic Waste Biomass. Chem. Eng. J. 2020, 390, 124607. [Google Scholar] [CrossRef]
- Plazzotta, S.; Calligaris, S.; Manzocco, L. Innovative Bioaerogel-like Materials from Fresh-Cut Salad Waste via Supercritical-CO2-Drying. Innov. Food Sci. Emerg. Technol. 2018, 47, 485–492. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Ni, Y. 3D Printing Using Plant-Derived Cellulose and Its Derivatives: A Review. Carbohydr. Polym. 2019, 203, 71–86. [Google Scholar] [CrossRef]
- Carlez, A.; Veciana-Nogues, T.; Cheftel, J.-C. Changes in Colour and Myoglobin of Minced Beef Meat Due to High Pressure Processing. LWT-Food Sci. Technol. 1995, 28, 528–538. [Google Scholar] [CrossRef]









| Aerogel Sample | CH (%) | LCMNF (%) | CMNF (%) | BT (%) |
|---|---|---|---|---|
| 100% CH | 100 | - | - | - |
| 1% LCMNF | 99 | 1 | ||
| 3% LCMNF | 97 | 3 | ||
| 5% LCMNF | 95 | 5 | ||
| 7% LCMNF | 93 | 7 | ||
| 10% LCMNF | 90 | 10 | ||
| 1% CMNF | 99 | - | 1 | - |
| 3% CMNF | 97 | 3 | ||
| 5% CMNF | 95 | 5 | ||
| 7% CMNF | 93 | 7 | ||
| 10% CMNF | 90 | 10 | ||
| Bioactive aerogel sample | ||||
| LCMNF + 0.3% BT | 95 | 5 | - | 0.3 |
| LCMNF + 0.7% BT | 0.7 | |||
| LCMNF + 1% BT | 1 | |||
| LCMNF + 2% BT | 2 | |||
| LCMNF + 5% BT | 5 | |||
| LCMNF + 10% BT | 10 | |||
| LCMNF + 20% BT | 20 | |||
| CMNF + 0.3% BT | 95 | - | 5 | 0.3 |
| CMNF + 0.7% BT | 0.7 | |||
| CMNF + 1% BT | 1 | |||
| CMNF + 2% BT | 2 | |||
| CMNF + 5% BT | 5 | |||
| CMNF + 10% BT | 10 | |||
| CMNF + 20% BT | 20 |
| η (%) a | CD (µeq/g) b | CC (µeq /g) c | Specific Surface (m2/g) | D (nm) d | Length (nm) | Aspect Ratio | |
|---|---|---|---|---|---|---|---|
| LCMNF | 48.06 ± 5.04 | 759.98 ± 38.38 | 146.11 ± 32.72 | 300 | 8.33 | 805.12 | 96.65 |
| CMNF | 58.763 ± 11.76 | 1349.02 ± 3.29 | 141.18 ± 23.57 | 591 | 4.23 | 562.17 | 132.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rincón, E.; Espinosa, E.; Pinillos, M.; Serrano, L. Bioactive Absorbent Chitosan Aerogels Reinforced with Bay Tree Pruning Waste Nanocellulose with Antioxidant Properties for Burger Meat Preservation. Polymers 2023, 15, 866. https://doi.org/10.3390/polym15040866
Rincón E, Espinosa E, Pinillos M, Serrano L. Bioactive Absorbent Chitosan Aerogels Reinforced with Bay Tree Pruning Waste Nanocellulose with Antioxidant Properties for Burger Meat Preservation. Polymers. 2023; 15(4):866. https://doi.org/10.3390/polym15040866
Chicago/Turabian StyleRincón, Esther, Eduardo Espinosa, María Pinillos, and Luis Serrano. 2023. "Bioactive Absorbent Chitosan Aerogels Reinforced with Bay Tree Pruning Waste Nanocellulose with Antioxidant Properties for Burger Meat Preservation" Polymers 15, no. 4: 866. https://doi.org/10.3390/polym15040866