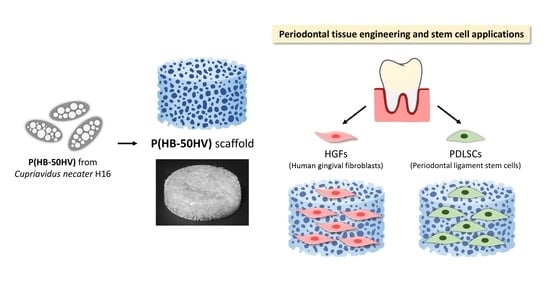
Microbial Poly(hydroxybutyrate-co-hydroxyvalerate) Scaffold for Periodontal Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Polymer Films
2.3. Fabrication and Characterization of Scaffolds
2.4. Compressive Mechanical Testing of Scaffolds
2.5. Evaluation of Protein Absorption on Scaffolds
2.6. Cell Culture
2.7. In Vitro Cell Proliferation Study
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Polymer Films
3.2. Characterization of Scaffolds
3.3. Mechanical Properties of Scaffolds
3.4. Cell Proliferation
3.5. Cell Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Swanson, W.B.; Yao, Y.; Mishina, Y. Novel approaches for periodontal tissue engineering. Genesis 2022, 60, e23499. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Xie, Y.; Chan, U.; Xu, T.; Huang, Y. Biomaterials and biotechnology for periodontal tissue regeneration: Recent advances and perspectives. J. Dent. Res. Dent. Clin. Dent. Prospects. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Chen, S.; Huang, X. Nanomaterials in Scaffolds for Periodontal Tissue Engineering: Frontiers and Prospects. Bioengineering 2022, 9, 431. [Google Scholar] [CrossRef]
- Rahman, S.U.; Nagrath, M.; Ponnusamy, S.; Arany, P.R. Nanoscale and Macroscale Scaffolds with Controlled-Release Polymeric Systems for Dental Craniomaxillofacial Tissue Engineering. Materials 2018, 11, 1478. [Google Scholar] [CrossRef]
- Diomede, F.; Gugliandolo, A.; Scionti, D.; Merciaro, I.; Cavalcanti, M.F.; Mazzon, E.; Trubiani, O. Biotherapeutic Effect of Gingival Stem Cells Conditioned Medium in Bone Tissue Restoration. Inter. J. Mol. Sci. 2018, 19, 329. [Google Scholar] [CrossRef]
- Gangolli, R.A.; Devlin, S.M.; Gerstenhaber, J.A.; Lelkes, P.I.; Yang, M. A Bilayered Poly (Lactic-Co-Glycolic Acid) Scaffold Provides Differential Cues for the Differentiation of Dental Pulp Stem Cells. Tissue. Eng. Part A 2019, 25, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.; Zheng, Y.; Liao, N.; Li, S.; Wang, Y.; Chen, Z.; Wei, L.; Zhao, S.; Mo, S. Mechanical force promotes the proliferation and extracellular matrix synthesis of human gingival fibroblasts cultured on 3D PLGA scaffolds via TGF-β expression. Mol. Med. Rep. 2019, 19, 2107–2114. [Google Scholar] [CrossRef] [Green Version]
- Baranowska-Korczyc, A.; Warowicka, A.; Jasiurkowska-Delaporte, M.; Grześkowiak, B.; Jarek, M.; Maciejewska, B.M.; Jurga-Stopa, J.; Jurga, S. Antimicrobial electrospun poly(ε-caprolactone) scaffolds for gingival fibroblast growth. RSC Adv. 2016, 6, 19647–19656. [Google Scholar] [CrossRef]
- Naik, C.; Srinath, N.; Ranganath, M.; Umashankar, D.; Gupta, H. Evaluation of polycaprolactone scaffold for guided bone regeneration in maxillary and mandibular defects: A clinical study. Nat. J. Maxillofac. Surg. 2020, 11, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Azaryan, E.; Hanafi-Bojd, M.Y.; Alemzadeh, E.; Emadian Razavi, F.; Naseri, M. Effect of PCL/nHAEA nanocomposite to osteo/odontogenic differentiation of dental pulp stem cells. BMC Oral Health 2022, 22, 505. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Inter. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Ibrahim, M.I.; Alsafadi, D.; Alamry, K.A.; Hussein, M.A. Properties and Applications of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Biocomposites. J. Polymer. Environ. 2021, 29, 1010–1030. [Google Scholar] [CrossRef]
- Köse, G.T.; Korkusuz, F.; Ozkul, A.; Soysal, Y.; Ozdemir, T.; Yildiz, C.; Hasirci, V. Tissue engineered cartilage on collagen and PHBV matrices. Biomaterials 2005, 26, 5187–5197. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.F.; Zare Karizi, S.; Hajati-Birgani, N.; Norouzi, S.; Khazeni, Z.; Hashemi, J.; Shafaghi, L.; Soleimanifar, F.; Mansour, R.N.; Enderami, S.E. PHBV nanofibers promotes insulin-producing cells differentiation of human induced pluripotent stem cells. Gene 2021, 768, 145333. [Google Scholar] [CrossRef]
- Amaro, L.; Correia, D.M.; Martins, P.M.; Botelho, G.; Carabineiro, S.A.C.; Ribeiro, C.; Lanceros-Mendez, S. Morphology Dependence Degradation of Electro- and Magnetoactive Poly(3-hydroxybutyrate-co-hydroxyvalerate) for Tissue Engineering Applications. Polymers 2020, 12, 953. [Google Scholar] [CrossRef]
- Napathorn, S.C. Biocompatibilities and biodegradation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)s produced by a model metabolic reaction-based system. BMC Microbiol. 2014, 14, 285. [Google Scholar] [CrossRef]
- Kim, H.S.; Chen, J.; Wu, L.P.; Wu, J.; Xiang, H.; Leong, K.W.; Han, J. Prevention of excessive scar formation using nanofibrous meshes made of biodegradable elastomer poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Tissue Eng. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Pramual, S.; Assavanig, A.; Bergkvist, M.; Batt, C.A.; Sunintaboon, P.; Lirdprapamongkol, K.; Svasti, J.; Niamsiri, N. Development and characterization of bio-derived polyhydroxyalkanoate nanoparticles as a delivery system for hydrophobic photodynamic therapy agents. J. Mater. Sci. Mater. Med. 2016, 27, 40. [Google Scholar] [CrossRef] [PubMed]
- Panith, N.; Assavanig, A.; Lertsiri, S.; Bergkvist, M.; Surarit, R.; Niamsiri, N. Development of tunable biodegradable polyhydroxyalkanoates microspheres for controlled delivery of tetracycline for treating periodontal disease. J. Appl. Polym. Sci. 2016, 133, 44128. [Google Scholar] [CrossRef]
- Pramual, S.; Lirdprapamongkol, K.; Svasti, J.; Bergkvist, M.; Jouan-Hureaux, V.; Arnoux, P.; Frochot, C.; Barberi-Heyob, M.; Niamsiri, N. Polymer-lipid-PEG hybrid nanoparticles as photosensitizer carrier for photodynamic therapy. J. Photochem. Photobiol. B 2017, 173, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Song, I.S.; Han, Y.S.; Lee, J.H.; Um, S.; Kim, H.Y.; Seo, B.M. Periodontal Ligament Stem Cells for Periodontal Regeneration. Curr. Oral Health Rep. 2015, 2, 236–244. [Google Scholar] [CrossRef]
- Intranuovo, F.; Gristina, R.; Brun, F.; Mohammadi, S.; Ceccone, G.; Sardella, E.; Rossi, F.; Tromba, G.; Favia, P. Plasma Modification of PCL Porous Scaffolds Fabricated by Solvent-Casting/Particulate-Leaching for Tissue Engineering. Plasma Process. Polym. 2014, 11, 184–195. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Jing, D.; Ding, J. “Wet-state” mechanical properties of three-dimensional polyester porous scaffolds. J. Biomed. Mater. Res. A 2006, 76, 264–271. [Google Scholar] [CrossRef]
- Aoki, N.; Akasaka, T.; Watari, F.; Yokoyama, A. Carbon nanotubes as scaffolds for cell culture and effect on cellular functions. Dent. Mater. J. 2007, 26, 178–185. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bäumchen, F.; Smeets, R.; Koch, D.; Gräber, H.G. The impact of defined polyglycolide scaffold structure on the proliferation of gingival fibroblasts in vitro: A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Shamala, T.R.; Divyashree, M.S.; Davis, R.; Kumari, K.S.; Vijayendra, S.V.; Raj, B. Production and characterization of bacterial polyhydroxyalkanoate copolymers and evaluation of their blends by fourier transform infrared spectroscopy and scanning electron microscopy. Indian J. Microbiol. 2009, 49, 251–258. [Google Scholar] [CrossRef]
- Porras, M.; Cubitto, M.; Villar, M. Quantitative Determination of intracellular PHA in Bacillus megaterium BBST4 strain Using Mid FTIR Spectroscopy. In Proceedings of the XIV SLAP/XII CIP 2014, Porto de Galinhas, Brazil, 12–16 October 2014. [Google Scholar] [CrossRef]
- Kumar, M.; Singhal, A.; Verma, P.K.; Thakur, I.S. Production and Characterization of Polyhydroxyalkanoate from Lignin Derivatives by Pandoraea sp. ISTKB. ACS Omega 2017, 2, 9156–9163. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H.; Yang, R.; Wu, Q.; Chen, G.-Q.; Zhang, Z.M. In situ FTIR study on melting and crystallization of polyhydroxyalkanoates. Polymer 2002, 43, 6893–6899. [Google Scholar] [CrossRef]
- Isak, I.; Patel, M.; Riddell, M.; West, M.; Bowers, T.; Wijeyekoon, S.; Lloyd, J. Quantification of polyhydroxyalkanoates in mixed and pure cultures biomass by Fourier transform infrared spectroscopy: Comparison of different approaches. Lett. Appl. Microbiol. 2016, 63, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Pashkuleva, I.; Reis, R.L.; Mano, J.F. Controlling Cell Behavior Through the Design of Polymer Surfaces. Small 2010, 6, 2208–2220. [Google Scholar] [CrossRef]
- Yu, D.G.; Lin, W.C.; Lin, C.H.; Yang, M.C. Cytocompatibility and antibacterial activity of a PHBV membrane with surface-immobilized water-soluble chitosan and chondroitin-6-sulfate. Macromol. Biosci. 2006, 6, 348–357. [Google Scholar] [CrossRef]
- Cheng, M.Q.; Wahafu, T.; Jiang, G.F.; Liu, W.; Qiao, Y.Q.; Peng, X.C.; Cheng, T.; Zhang, X.L.; He, G.; Liu, X.Y. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci. Rep. 2016, 6, 24134. [Google Scholar] [CrossRef]
- Yazid, F. Scaffold Selection for Tissue Engineering in Dentistry. Med. Health 2020, 15, 34–53. [Google Scholar] [CrossRef]
- Verlinden, R.A.J.; Hill, D.J.; Kenward, M.A.; Williams, C.D.; Radecka, I. Bacterial synthesis of biodegradable polyhydroxyalkanoates. J. Appl. Microbiol. 2007, 102, 1437–1449. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Passerini, N.; Craig, D.Q. An investigation into the effects of residual water on the glass transition temperature of polylactide microspheres using modulated temperature DSC. J. Control Release 2001, 73, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Xu, J.; Li, L.; et al. Effects of Matrix Stiffness on the Morphology, Adhesion, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.C.; Rohman, G.; Southgate, J.; Cameron, N.R. The relationship between the mechanical properties and cell behaviour on PLGA and PCL scaffolds for bladder tissue engineering. Biomaterials 2009, 30, 1321–1328. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Wang, C.; Chen, S. In vitro behaviour of human gingival fibroblasts cultured on 3D-printed titanium alloy with hydrogenated TiO2 nanotubes. J. Mater. Sci. Mater. Med. 2022, 33, 27. [Google Scholar] [CrossRef]
- Sugiura, R.; Hamano, S.; Tomokiyo, A.; Hasegawa, D.; Yoshida, S.; Sugii, H.; Fujino, S.; Adachi, O.; Kadowaki, M.; Yamashita, D.; et al. PAX9 Is Involved in Periodontal Ligament Stem Cell-like Differentiation of Human-Induced Pluripotent Stem Cells by Regulating Extracellular Matrix. Biomedicines 2022, 10, 2366. [Google Scholar] [CrossRef]







| Type of Polymers | Water Contact Angle (°) |
|---|---|
| PHB | 70.2 ± 3.5 a |
| P(HB-12HV) | 67.9 ± 2.1 a |
| P(HB-50HV) | 76.8 ± 1.8 b |
| PCL | 81.4 ± 1.8 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuegyod, S.; Pramual, S.; Wattanavichean, N.; Assawajaruwan, S.; Amornsakchai, T.; Sukho, P.; Svasti, J.; Surarit, R.; Niamsiri, N. Microbial Poly(hydroxybutyrate-co-hydroxyvalerate) Scaffold for Periodontal Tissue Engineering. Polymers 2023, 15, 855. https://doi.org/10.3390/polym15040855
Phuegyod S, Pramual S, Wattanavichean N, Assawajaruwan S, Amornsakchai T, Sukho P, Svasti J, Surarit R, Niamsiri N. Microbial Poly(hydroxybutyrate-co-hydroxyvalerate) Scaffold for Periodontal Tissue Engineering. Polymers. 2023; 15(4):855. https://doi.org/10.3390/polym15040855
Chicago/Turabian StylePhuegyod, Seubsakul, Sasivimon Pramual, Nungnit Wattanavichean, Supasuda Assawajaruwan, Taweechai Amornsakchai, Panithi Sukho, Jisnuson Svasti, Rudee Surarit, and Nuttawee Niamsiri. 2023. "Microbial Poly(hydroxybutyrate-co-hydroxyvalerate) Scaffold for Periodontal Tissue Engineering" Polymers 15, no. 4: 855. https://doi.org/10.3390/polym15040855