Synergistic Effects of 1-Octyl-3-Methylimidazolium Hexafluorophosphate and Cellulose Nanocrystals on Improving Polyacrylate Waterborne Anti-Corrosion Coatings
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Materials
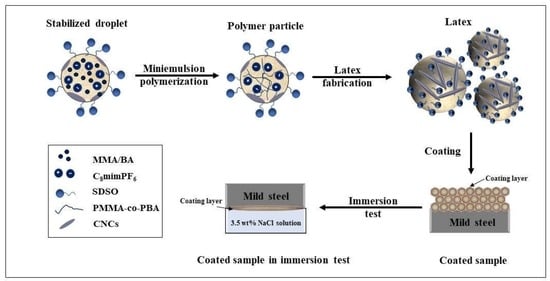
2.2. Preparation of Mini Emulsion
2.3. Mini Emulsion Polymerization
2.4. Coating Film Characterization
2.4.1. Testing Sample Preparation
2.4.2. FTIR
2.4.3. Surface Morphology
2.4.4. Wettability
2.4.5. Electrochemical Techniques
3. Results and Discussion
3.1. Anti-Corrosion Performance Evaluation of Copolymer Coating
3.1.1. Open Circuit Potential (OCP)
3.1.2. Electrochemical Impedance Spectroscopy (EIS)
3.1.3. Tafel Polarization Plot
3.1.4. Morphology after Immersion Tests
3.2. Mechanism of C8mimPF6 and CNCs in PMMA-co-PBA Anti-Corrosion Coating
3.2.1. Status Identification of C8mimPF6 and CNCs in PMMA-co-PBA Coating
3.2.2. Wettability of Copolymer Coating
3.2.3. Synergistic Effect of C8mimPF6 and CNCs in PMMA-co-PBA Anti-Corrosion Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Hu, B.; Chen, G.Z. The role of 1-octyl-3-methylimidazolium hexafluorophosphate in anticorrosion coating formula development. J. Saudi Chem. Soc. 2022, 26, 101446. [Google Scholar] [CrossRef]
- Likhanova, N.V.; Domínguez-Aguilar, M.A.; Olivares-Xometl, O.; Nava-Entzana, N.; Arce, E.; Dorantes, H. The effect of ionic liquids with imidazolium and pyridinium cations on the corrosion inhibition of mild steel in acidic environment. Corros. Sci. 2010, 52, 2088–2097. [Google Scholar] [CrossRef]
- Ding, J.; Shi, S.; Yu, H. Study on modification of lignin as dispersant of aqueous graphene suspension and corrosion performance in waterborne G/epoxy coating. Int. J. Adv. Eng. Res. Sci. 2016, 3, 101–112. [Google Scholar] [CrossRef]
- Carretti, E.; Dei, L. Physicochemical characterization of acrylic polymeric resins coating porous materials of artistic interest. Prog. Org. Coat. 2004, 49, 282–289. [Google Scholar] [CrossRef]
- Du, B.; Chen, F.; Luo, R.; Zhou, S.; Wu, Z. Synthesis and characterization of nano-TiO2/SiO2-acrylic composite resin. Adv. Mater. Sci. Eng. 2019, 2019, 6318623. [Google Scholar] [CrossRef]
- Zhan, Y.; Meng, Y.; Li, Y.; Zhang, C.; Xie, Q.; Wei, S.; Lavorgna, M.; Chen, Z. Poly (vinyl alcohol)/reduced graphene oxide multilayered coatings: The effect of filler content on gas barrier and surface resistivity properties. Compos. Commun. 2021, 24, 100670. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2008, 10, 162–165. [Google Scholar] [CrossRef]
- Follain, N.; Belbekhouche, S.; Bras, J.; Siqueira, G.; Marais, S.; Dufresne, A. Water transport properties of bio-nanocomposites reinforced by Luffa cylindrica cellulose nanocrystals. J. Membr. Sci. 2013, 427, 218–229. [Google Scholar] [CrossRef]
- He, Y.; Boluk, Y.; Pan, J.; Ahniyaz, A.; Deltin, T.; Claesson, P.M. Corrosion protective properties of cellulose nanocrystals reinforced waterborne acrylate-based composite coating. Corros. Sci. 2019, 155, 186–194. [Google Scholar] [CrossRef]
- Liu, P.; Guo, X.; Nan, F.; Duan, Y.; Zhang, J. Modifying mechanical, optical properties and thermal processability of iridescent cellulose nanocrystal films using ionic liquid. ACS Appl. Mater. Interfaces 2017, 9, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, J.; Liu, Y.; Shu, J.; Wu, H.; Wang, Z.; Pan, X.; Zhang, N.; Zhou, L.; Zhang, J. Ionic liquids grafted cellulose nanocrystals for high-strength and toughness PVA nanocomposite. ACS Appl. Mater. Interfaces 2020, 12, 38796–38804. [Google Scholar] [CrossRef]
- Wahib, S.A.; Da’na, D.A.; Zaouri, N.; Hijji, Y.M.; Al-Ghouti, M.A. Adsorption and recovery of lithium ions from groundwater using date pits impregnated with cellulose nanocrystals and ionic liquid. J. Hazard. Mater. 2022, 421, 126657. [Google Scholar] [CrossRef] [PubMed]
- Tait, W.S. Electrochemical corrosion basics. In Handbook of Environmental Degradation of Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 97–115. [Google Scholar]
- Ghasemi-Kahrizsangi, A.; Shariatpanahi, H.; Neshati, J.; Akbarinezhad, E. Corrosion behavior of modified nano carbon black/epoxy coating in accelerated conditions. Appl. Surf. Sci. 2015, 331, 115–126. [Google Scholar] [CrossRef]
- Qiu, S.; Li, W.; Zheng, W.; Zhao, H.; Wang, L. Synergistic effect of polypyrrole-intercalated graphene for enhanced corrosion protection of aqueous coating in 3.5% NaCl solution. ACS Appl. Mater. Interfaces 2017, 9, 34294–34304. [Google Scholar] [CrossRef] [PubMed]
- Brasher, D.; Kingsbury, A. Electrical measurements in the study of immersed paint coatings on metal. I. Comparison between capacitance and gravimetric methods of estimating water-uptake. J. Appl. Chem. 1954, 4, 62–72. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Orazem, M.E.; Pébère, N.; Tribollet, B. Enhanced graphical representation of electrochemical impedance data. J. Electrochem. Soc. 2006, 153, B129. [Google Scholar] [CrossRef]
- Bagal, N.S.; Kathavate, V.S.; Deshpande, P.P. Nano-TiO2 Phosphate Conversion Coatings–A Chemical Approach. Electrochem. Energy Technol. 2018, 4, 47–54. [Google Scholar] [CrossRef]
- Kathavate, V.S.; Pawar, D.; Bagal, N.; Deshpande, P. Role of nano ZnO particles in the electrodeposition and growth mechanism of phosphate coatings for enhancing the anti-corrosive performance of low carbon steel in 3.5% NaCl aqueous solution. J. Alloys Compd. 2020, 823, 153812. [Google Scholar] [CrossRef]
- Holm, S.; Holm, T.; Martinsen, Ø.G. Simple circuit equivalents for the constant phase element. PLoS ONE 2021, 16, e0248786. [Google Scholar] [CrossRef]
- Cano, E.; Lafuente, D.; Bastidas, D.M. Use of EIS for the evaluation of the protective properties of coatings for metallic cultural heritage: A review. J. Solid State Electrochem. 2010, 14, 381–391. [Google Scholar] [CrossRef]
- Mostafaei, A.; Nasirpouri, F. Epoxy/polyaniline–ZnO nanorods hybrid nanocomposite coatings: Synthesis, characterization and corrosion protection performance of conducting paints. Prog. Org. Coat. 2014, 77, 146–159. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Li, W.; Cui, Y.; Yang, T. Synthesis of graphene oxide-based sulfonated oligoanilines coatings for synergistically enhanced corrosion protection in 3.5% NaCl solution. ACS Appl. Mater. Interfaces 2017, 9, 4034–4043. [Google Scholar] [CrossRef]
- Lewis, O.; Critchlow, G.; Wilcox, G.; de Zeeuw, A.; Sander, J. A study of the corrosion resistance of a waterborne acrylic coating modified with nano-sized titanium dioxide. Prog. Org. Coat. 2012, 73, 88–94. [Google Scholar] [CrossRef]
- Malmberg, C.; Maryott, A. Dielectric Constant of Water from 00 to 1000 C. J. Res. Natl. Bur. Stand. 1956, 56, 1. [Google Scholar] [CrossRef]
- Fu, L.-H.; Qi, C.; Ma, M.-G.; Wan, P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 1541–1562. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Gui, H.; Guan, G.; Zhou, M.; Guo, Q.; Tan, M.Y. Molecular design and copolymerization to enhance the anti-corrosion performance of waterborne acrylic coatings. Prog. Org. Coat. 2021, 153, 106140. [Google Scholar] [CrossRef]
- Frankel, G. Fundamentals of corrosion kinetics. In Active Protective Coatings; Springer: Berlin/Heidelberg, Germany, 2016; pp. 17–32. [Google Scholar]
- Hamidon, T.S.; Hussin, M.H. Susceptibility of hybrid sol-gel (TEOS-APTES) doped with caffeine as potent corrosion protective coatings for mild steel in 3.5 wt.% NaCl. Prog. Org. Coat. 2020, 140, 105478. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Amine cured double Schiff base epoxy as efficient anticorrosive coating materials for protection of mild steel in 3.5% NaCl medium. J. Mol. Liq. 2019, 278, 521–535. [Google Scholar] [CrossRef]
- Weng, C.J.; Huang, J.Y.; Huang, K.Y.; Jhuo, Y.S.; Tsai, M.H.; Yeh, J.M. Advanced anticorrosive coatings prepared from electroactive polyimide–TiO hybrid nanocomposite materials. Electrochim. Acta 2010, 55, 8430–8438. [Google Scholar] [CrossRef]
- Yeh, J.M.; Huang, H.Y.; Chen, C.L.; Su, W.F.; Yu, Y.H. Siloxane-modified epoxy resin–clay nanocomposite coatings with advanced anticorrosive properties prepared by a solution dispersion approach. Surf. Coat. Technol. 2006, 200, 2753–2763. [Google Scholar] [CrossRef]
- Cai, K.; Zuo, S.; Luo, S.; Yao, C.; Liu, W.; Ma, J.; Maoa, H.; Li, Z. Preparation of polyaniline/graphene composites with excellent anti-corrosion properties and their application in waterborne polyurethane anticorrosive coatings. RSC Adv. 2016, 6, 95965–95972. [Google Scholar] [CrossRef]
- Iimori, T.; Iwahashi, T.; Kanai, K.; Seki, K.; Sung, J.; Kim, D.; Hamaguchi, H.-o.; Ouchi, Y. Local Structure at the Air/Liquid Interface of Room-Temperature Ionic Liquids Probed by Infrared− Visible Sum Frequency Generation Vibrational Spectroscopy: 1-Alkyl-3-methylimidazolium Tetrafluoroborates. J. Phys. Chem. B 2007, 111, 4860–4866. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R.; Nazri, M.; Nazri, G.; Camargo, A.; Trsic, M. Vibrational spectra and ion-pair properties of lithium hexafluorophosphate in ethylene carbonate based mixed-solvent systems for lithium batteries. J. Solut. Chem. 2000, 29, 1047–1060. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, J.; Lu, R.; Zhang, S.; Zhou, W.; Gao, H. Dispersive micro-solid phase extraction based on self-assembling, ionic liquid-coated magnetic particles for the determination of clofentezine and chlorfenapyr in environmental water samples. Analyst 2013, 138, 6834–6843. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Wulandari, W.; Rochliadi, A.; Arcana, I. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 107, p. 012045. [Google Scholar]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues–Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Lamaming, J.; Hashim, R.; Sulaiman, O.; Leh, C.P.; Sugimoto, T.; Nordin, N.A. Cellulose nanocrystals isolated from oil palm trunk. Carbohydr. Polym. 2015, 127, 202–208. [Google Scholar] [CrossRef]
- Duan, G.; Zhang, C.; Li, A.; Yang, X.; Lu, L.; Wang, X. Preparation and characterization of mesoporous zirconia made by using a poly (methyl methacrylate) template. Nanoscale Res. Lett. 2008, 3, 118. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, S.; Leen, K.H.; Kumutha, K.; Arof, A. FTIR studies of PVC/PMMA blend based polymer electrolytes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 66, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 2009, 102, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A.K. Formulation and characterization of hybrid polymeric/ZnO nanocomposite coatings with remarkable anti-corrosion and hydrophobic characteristics. J. Coat. Technol. Res. 2016, 13, 921–930. [Google Scholar] [CrossRef]
- Nakae, H.; Inui, R.; Hirata, Y.; Saito, H. Effects of surface roughness on wettability. Acta Mater. 1998, 46, 2313–2318. [Google Scholar] [CrossRef]
- Mantel, M.; Wightman, J. Influence of the surface chemistry on the wettability of stainless steel. Surf. Interface Anal. 1994, 21, 595–605. [Google Scholar] [CrossRef]
- Pech-Canul, M.; Bartolo-Perez, P. Inhibition effects of N-phosphono-methyl-glycine/Zn2+ mixtures on corrosion of steel in neutral chloride solutions. Surf. Coat. Technol. 2004, 184, 133–140. [Google Scholar] [CrossRef]
- Ma, L.; Chen, F.; Li, Z.; Gan, M.; Yan, J.; Wei, S.; Bai, Y.; Zeng, J. Preparation and anticorrosion property of poly (2, 3-dimethylaniline) modified by nano-SiO2. Compos. Part B Eng. 2014, 58, 54–58. [Google Scholar] [CrossRef]
- Matin, E.; Attar, M.; Ramezanzadeh, B. Investigation of corrosion protection properties of an epoxy nanocomposite loaded with polysiloxane surface modified nanosilica particles on the steel substrate. Prog. Org. Coat. 2015, 78, 395–403. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H.; Mu, G. Synergistic inhibition effect of rare earth cerium (IV) ion and anionic surfactant on the corrosion of cold rolled steel in H2SO4 solution. Corros. Sci. 2008, 50, 2635–2645. [Google Scholar] [CrossRef]
- Bentiss, F.; Lebrini, M.; Lagrenée, M. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2, 5-bis (n-thienyl)-1, 3, 4-thiadiazoles/hydrochloric acid system. Corros. Sci. 2005, 47, 2915–2931. [Google Scholar] [CrossRef]
- Velusamy, S.; Sakthivel, S.; Neelakantan, L.; Sangwai, J.S. Imidazolium-based ionic liquids as an anticorrosive agent for completion fluid design. J. Earth Sci. 2017, 28, 949–961. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Imidazolium grafted cellulose nanocrystals for ion exchange applications. Chem. Commun. 2011, 47, 4177–4179. [Google Scholar] [CrossRef]












| Sample | Ecorr (mV) | Icorr (µA/cm2) | ba (V·dec−1) | bc (V·dec−1) | Inhibition Efficiency | Rcorr (µm per Year) |
|---|---|---|---|---|---|---|
| Bare steel | −646 | 9.4 | 61 | −334 | 109 | |
| Blank | −605 | 4.6 | 82 | −222 | 51% | 54 |
| C8mimPF6 | −534 | 2.9 | 103 | −305 | 70% | 34 |
| CNCs | −550 | 3.1 | 138 | −287 | 67% | 36 |
| C8mimPF6-CNCs | −467 | 0.5 | 173 | −112 | 94% | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Hu, B.; Yu, H.; Chen, G.Z. Synergistic Effects of 1-Octyl-3-Methylimidazolium Hexafluorophosphate and Cellulose Nanocrystals on Improving Polyacrylate Waterborne Anti-Corrosion Coatings. Polymers 2023, 15, 810. https://doi.org/10.3390/polym15040810
Wang Z, Hu B, Yu H, Chen GZ. Synergistic Effects of 1-Octyl-3-Methylimidazolium Hexafluorophosphate and Cellulose Nanocrystals on Improving Polyacrylate Waterborne Anti-Corrosion Coatings. Polymers. 2023; 15(4):810. https://doi.org/10.3390/polym15040810
Chicago/Turabian StyleWang, Zeping, Binjie Hu, Haibin Yu, and George Zheng Chen. 2023. "Synergistic Effects of 1-Octyl-3-Methylimidazolium Hexafluorophosphate and Cellulose Nanocrystals on Improving Polyacrylate Waterborne Anti-Corrosion Coatings" Polymers 15, no. 4: 810. https://doi.org/10.3390/polym15040810