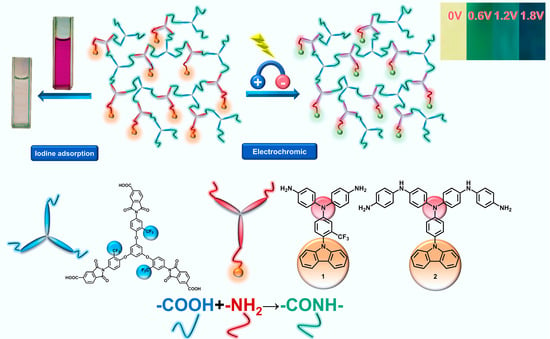
Preparation of Novel Nitrogen-Rich Fluorinated Hyperbranched Poly(amide-imide) and Evaluation of Its Electrochromic Properties and Iodine Adsorption Behavior
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of Triacid Monomer (BTTFTDCA) and Pendant Monomers
2.2. Structural Characterization of Poly(amide-imide) (PAI-1 and PAI-2)
2.3. Thermal Properties of the PAIs
2.4. Inherent Viscosity, GPC Value and Solubility of the PAIs
2.5. Film Forming Properties of the PAIs
2.6. Electrochemical Characteristics
2.7. Electrochromic Characteristics
2.8. Adsorption of Iodide Ion Solutions
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jikei, M.; Kakimoto, M.-A. Hyperbranched polymers: A promising new class of materials. Prog. Polym. Sci. 2001, 26, 1233–1285. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.; Frey, H. Linear–dendritic block copolymers: The state of the art and exciting perspectives. Prog. Polym. Sci. 2011, 36, 1–52. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.; Zhang, D. Synthesis and application of epoxy-ended hyperbranched polymers. Chem. Eng. J. 2018, 343, 283–302. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Yu, H.; Zhao, Y.; Sun, R.; Jing, G.; Huang, J.; Khalid, H.; Abbasi, N.M.; Akram, M. Synthesis and application of polyethylene-based functionalized hyperbranched polymers. Prog. Polym. Sci. 2015, 45, 23–43. [Google Scholar] [CrossRef]
- Zheng, B.; Li, Y.; Tao, F.; Cui, Y.; Li, T. Enhanced superquenching of the hyperbranched conjugated polymer for the detection of nitroaromatic explosives. Sens. Actuators B 2017, 241, 357–363. [Google Scholar] [CrossRef]
- Ma, X.; Tao, F.; Zhang, Y.; Li, T.; Raymo, F.M.; Cui, Y. Detection of nitroaromatic explosives by a 3D hyperbranched σ–π conjugated polymer based on a POSS scaffold. J. Mater. Chem. A 2017, 5, 14343–14354. [Google Scholar] [CrossRef]
- Liu, G.; Chen, P.; Tang, R.; Li, Z. Synthesis and characterization of dendronized hyperbranched polymers through the “A3+B2” approach. Sci. China Chem. 2016, 59, 1561–1567. [Google Scholar] [CrossRef]
- Yamamoto, K.; Suemasa, D.; Masuda, K.; Aita, K.; Endo, T. Hyperbranched Triphenylamine Polymer for UltraFast Battery Cathode. ACS Appl. Mater. Interfaces 2018, 10, 6346–6353. [Google Scholar] [CrossRef]
- Wei, Q.; Pötzsch, R.; Liu, X.; Komber, H.; Kiriy, A.; Voit, B.; Will, P.; Lenk, S.; Reineke, S. Hyperbranched Polymers with High Transparency and Inherent High Refractive Index for Application in Organic Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2545–2553. [Google Scholar] [CrossRef]
- Chen, S.; Feng, F.; Yin, Y.; Che, H.; Liao, X.-Z.; Ma, Z.-F. A solid polymer electrolyte based on star-like hyperbranched β-cyclodextrin for all-solid-state sodium batteries. J. Power Sources 2018, 399, 363–371. [Google Scholar] [CrossRef]
- Han, Y.; Sun, M.; Fei, Z.; Bo, Z. Hyperbranched polymer-cored star polyfluorenes as blue light-emitting materials. Chin. Sci. Bull. 2008, 53, 2770–2776. [Google Scholar] [CrossRef]
- Feng, H.; Ma, W.; Cui, Z.-K.; Liu, X.; Gu, J.; Lin, S.; Zhuang, Q. Core/shell-structured hyperbranched aromatic polyamide functionalized graphene nanosheets-poly(p-phenylene benzobisoxazole) nanocomposite films with improved dielectric properties and thermostability. J. Mater. Chem. A 2017, 5, 8705–8713. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Beraa, A.; Laurent, R.; Delavaux-Nicot, B.; Hajjaji, M. Dendrimers and hyper-branched polymers interacting with clays: Fruitful associations for functional materials. J. Mater. Chem. A 2019, 7, 19634–19650. [Google Scholar] [CrossRef]
- Flouda, P.; Bukharina, D.; Pierce, K.J.; Stryutsky, A.V.; Shevchenko, V.V.; Tsukruk, V.V. Flexible Sustained Ionogels with Ionic Hyperbranched Polymers for Enhanced Ion-Conduction and Energy Storage. ACS Appl. Mater. Interfaces 2022, 14, 27028–27039. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-W.; Sato, T.; Zhang, J.; Moriyama, S.; Higuchi, M. Three-Dimensional Fe(II)-based Metallo-Supramolecular Polymers with Electrochromic Properties of Quick Switching, Large Contrast, and High Coloration Efficiency. ACS Appl. Mater. Interfaces 2014, 6, 9118–9125. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Chakraborty, C. Interfacial Coordination Nanosheet Based on Nonconjugated Three-Arm Terpyridine: A Highly Color-Efficient Electrochromic Material to Converge Fast Switching with Long Optical Memory. ACS Appl. Mater. Interfaces 2020, 12, 35181–35192. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, X.; Dou, S.; Zhang, L.; Zhang, H.; Lv, H.; Wang, L.; Zhao, J.; Li, Y. A comprehensive study of electrochromic device with variable infrared emissivity based on polyaniline conducting polymer. Sol. Energy Mater. Sol. Cells 2017, 170, 120–126. [Google Scholar] [CrossRef]
- Frolov, D.; Petrov, M.; Makhaeva, E.; Keshtov, M.; Khokhlov, A. Electrochromic behavior of poly(pyridinium triflates) films: Electrolyte ions influence. Synth. Met. 2018, 239, 29–35. [Google Scholar] [CrossRef]
- Avais, M.; Chattopadhyay, S. Hierarchical Porous Polymers via a Microgel Intermediate: Green Synthesis and Applications toward the Removal of Pollutants. ACS Appl. Polym. Mater. 2021, 3, 789–800. [Google Scholar] [CrossRef]
- Avais, M.; Kumari, S.; Chattopadhyay, S. Degradable and processable polymer monoliths with open-pore porosity for selective CO2 and iodine adsorption. Soft Matter 2021, 17, 6383–6393. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, L.; Chen, H.; Jia, Y.; Ma, Y. Electropolymerized Triphenylamine Network Films for High-Performance Transparent to Black Electrochromism and Capacitance. Adv. Opt. Mater. 2023, 11, 2201572. [Google Scholar] [CrossRef]
- Cai, W.; Xiao, T.; Niu, H.; Bai, X.; Zhang, Y.; Wang, C.; Wang, W.; Qi, H. Novel electrochromic triphenylamine-based polyamides containing quinolin-8-yloxy group as probes for metal ions. Sens. Actuators B 2017, 252, 330–339. [Google Scholar] [CrossRef]
- Shao, Y.-J.; Tu, M.-H.; Liou, G.-S. Unprecedented facile approach of multiple amino-substituted triphenylamine derivatives for electrochromic devices with extremely high coloration efficiency and unexpected redox stability. Chem. Eng. J. 2023, 466, 143003. [Google Scholar] [CrossRef]
- Yu, X.; Chang, M.; Chen, W.; Liang, D.; Lu, X.; Zhou, G. Colorless-to-Black Electrochromism from Binary Electrochromes toward Multifunctional Displays. ACS Appl. Mater. Interfaces 2020, 12, 39505–39514. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Li, H.; Wang, J.-R.; Li, P.-Z.; Zhao, Y. From Supramolecular Organic Cages to Porous Covalent Organic Frameworks for Enhancing Iodine Adsorption Capability by Fully Exposed Nitrogen-Rich Sites. Small 2023, 19, 2301998. [Google Scholar] [CrossRef]
- Cheng, K.; Li, H.; Li, Z.; Li, P.-Z.; Zhao, Y. Linking Nitrogen-Rich Organic Cages into Isoreticular Covalent Organic Frameworks for Enhancing Iodine Adsorption Capability. ACS Mater. Lett. 2023, 5, 1546–1555. [Google Scholar] [CrossRef]
- Zhang, L.; Zhan, W.; Dong, Y.; Yang, T.; Zhang, C.; Ouyang, M.; Li, W. Liquid/Liquid Interfacial Suzuki Polymerization Prepared Novel Triphenylamine-Based Conjugated Polymer Films with Excellent Electrochromic Properties. ACS Appl. Mater. Interfaces 2021, 13, 20810–20820. [Google Scholar] [CrossRef]
- Zheng, R.; Fan, Y.; Wang, Y.; Wan, Z.; Jia, C.; Weng, X.; Xie, J.; Deng, L. A bifunctional triphenylamine-based electrochromic polymer with excellent self-healing performance. Electrochim. Acta 2018, 286, 296–303. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Li, H.; Li, X.-M. Imine-linked covalent organic frameworks with stable and microporous structure for effective carbon dioxide and iodine uptake. Microporous Mesoporous Mater. 2023, 349, 112419. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Ren, B.; Zhu, X.; Zhou, W.; Li, W. Novel low color poly(ester imides) with triphenylamine and carbazole substituents for electrochromic applications. Dye. Pigm. 2019, 162, 232–242. [Google Scholar] [CrossRef]
- Liu, Y.; Pang, L.; Liu, T.; Guo, J.; Wang, J.; Li, W. Novel triphenylamine polyazomethines bearing carbazole and trifluoromethyl substituents: Preparation and electrochromic behavior. Dye. Pigm. 2020, 173, 107921. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Pang, L.; Guo, J.; Wang, J.; Qi, D.; Li, W.; Shen, K. Novel triphenylamine polyamides bearing carbazole and aniline substituents for multi-colored electrochromic applications. Dye. Pigm. 2020, 173, 107995. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.; Wang, J.; Zhu, X.; Qi, D.; Li, W.; Shen, K. A novel family of optically transparent fluorinated hyperbranched polyimides with long linear backbones and bulky substituents. Eur. Polym. J. 2020, 125, 109526. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Guo, J.; Qi, D.; Li, W.; Shen, K. Novel fluorinated long linear segment hyperbranched polyimides bearing various pendant substituents for applications as optical materials. Polymer 2020, 190, 122216. [Google Scholar] [CrossRef]
- Li, L.; Sun, Z.; Yang, A.; Zhang, X.; Zhu, X.; Li, W.; Liu, Y.; Luan, J. A Novel Azo-Linked Polymer Bearing Trifluoromethyl Groups for I2 Capture. Macromol. Rapid Commun. 2023, 44, 2200982. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, D.; Guan, S.; Jiang, W.; Jiang, Z.; Gao, W.; Zhang, D. Fluorinated Hyperbranched Polyimide for Optical Waveguides. Macromol. Rapid Commun. 2007, 28, 252–259. [Google Scholar] [CrossRef]
- Wu, H.-L.; Ma, C.-C.M.; Li, C.-H.; Lee, T.-M.; Chen, C.-Y.; Chiang, C.-L.; Wu, C. Sulfonated poly(ether ether ketone)/poly(amide imide) polymer blends for proton conducting membrane. J. Membr. Sci. 2006, 280, 501–508. [Google Scholar] [CrossRef]
- Gao, H.; Yan, C.; Guan, S.; Jiang, Z. Hyperbranched fluorinated polyimides with tunable refractive indices for optical waveguide applications. Polymer 2010, 51, 694–701. [Google Scholar] [CrossRef]
- Hawker, C.J.; Chu, F. Hyperbranched Poly(ether ketones): Manipulation of Structure and Physical Properties. Macromolecules 1996, 29, 4370–4380. [Google Scholar] [CrossRef]
- Hawker, C.J.; Lee, R.; Frechet, J.M.J. One-step synthesis of hyperbranched dendritic polyesters. J. Am. Chem. Soc. 1991, 113, 4583–4588. [Google Scholar] [CrossRef]
- Xu, W.; Yang, G.; Jin, L.; Liu, J.; Zhang, Y.; Zhang, Z.; Jiang, Z. High-k Polymer Nanocomposites Filled with Hyperbranched Phthalocyanine-Coated BaTiO3 for High-Temperature and Elevated Field Applications. ACS Appl. Mater. Interfaces 2018, 10, 11233–11241. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, J.; Wang, J.; Wu, Y.; Qin, A.; Tang, B.Z. Preparation of Multifunctional Hyperbranched Poly(β-aminoacrylate)s by Spontaneous Amino-yne Click Polymerization. Macromolecules 2020, 53, 5248–5254. [Google Scholar] [CrossRef]
- Yang, M.-L.; Wu, Y.-X.; Liu, Y.; Qiu, J.-J.; Liu, C.-M. A novel bio-based AB2 monomer for preparing hyperbranched polyamides derived from levulinic acid and furfurylamine. Polym. Chem. 2019, 10, 6217–6226. [Google Scholar] [CrossRef]
- Li, W.; Yuan, F.; Xu, N.; Mei, S.; Chen, Z.; Zhang, C. Triphenylamine-triazine polymer materials obtained by electrochemical polymerization: Electrochemistry stability, anions trapping behavior and electrochromic-supercapacitor application. Electrochim. Acta 2021, 384, 138344. [Google Scholar] [CrossRef]
- Querfeld, R.; Pasi, A.-E.; Shozugawa, K.; Vockenhuber, C.; Synal, H.-A.; Steier, P.; Steinhauser, G. Radionuclides in surface waters around the damaged Fukushima Daiichi NPP one month after the accident: Evidence of significant tritium release into the environment. Sci. Total Environ. 2019, 689, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.; He, X.; Abu-Reziq, R.; Pan, C.; Tang, J.; Yu, G. Fluorinated covalent triazine frameworks for effective CH4 separation and iodine vapor uptake. Sep. Purif. Technol. 2022, 290, 120857. [Google Scholar] [CrossRef]
- Xu, J.; Xie, W.; Yao, C.; Xu, G.; Zhang, S.; Xu, Y. Preparation of sulfur-containing conjugated microporous polymer for adsorbing iodine and Fe3+ sensing. J. Environ. Chem. Eng. 2021, 9, 106399. [Google Scholar] [CrossRef]
- Xie, W.; Cui, D.; Zhang, S.-R.; Xu, Y.-H.; Jiang, D.-L. Iodine capture in porous organic polymers and metal–organic frameworks materials. Mater. Horiz. 2019, 6, 1571–1595. [Google Scholar] [CrossRef]
- Yin, Q.; Si, L.; Wang, R.; Zhao, Z.; Li, H.; Wen, Z. DFT study on the effect of functional groups of carbonaceous surface on ammonium adsorption from water. Chemosphere 2022, 287, 132294. [Google Scholar] [CrossRef]














| Polymers | DSC | TGA | ||
|---|---|---|---|---|
| T a (°C) | T5% b (°C) | T10% c (°C) | Char Yied d (%) | |
| PAI-1 | 256 | 574 | 608 | 56.2 |
| PAI-2 | 261 | 514 | 564 | 60.7 |
| Polymers | Inherent Viscosity | GPC Data | Solvents | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ninh a (dL/g) | Mw b (g/mol) | Mw/Mn b | DMSO | NMP | DMAc | DMF | THF | CHCl3 | EtOAc | EtOH | |
| PAI-1 | 0.41 | 6.2 × 104 | 2.3 | ++ | ++ | ++ | ++ | + | - | - | - |
| PAI-2 | 0.43 | 6.5 × 104 | 2.1 | ++ | ++ | ++ | ++ | + | - | - | - |
| Index | Thin Film (λ/nm) | Oxidation Potential a (V) | Eg b (eV) | HOMO c (eV) | LUMO d (eV) | |||
|---|---|---|---|---|---|---|---|---|
| Abs.max | Abs.onset | Eonset | EOX11/2 | EOX21/2 | ||||
| PAI-1 | 337 | 406 | 0.61 | 1.16 | 1.71 | 3.05 | 4.83 | 1.78 |
| PAI-2 | 337 | 391 | 0.63 | 1.07 | 1.78 | 3.17 | 4.85 | 1.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Yang, W.; Zhang, X.; Zhu, X.; Luan, J.; Li, W.; Liu, Y. Preparation of Novel Nitrogen-Rich Fluorinated Hyperbranched Poly(amide-imide) and Evaluation of Its Electrochromic Properties and Iodine Adsorption Behavior. Polymers 2023, 15, 4537. https://doi.org/10.3390/polym15234537
Sun Z, Yang W, Zhang X, Zhu X, Luan J, Li W, Liu Y. Preparation of Novel Nitrogen-Rich Fluorinated Hyperbranched Poly(amide-imide) and Evaluation of Its Electrochromic Properties and Iodine Adsorption Behavior. Polymers. 2023; 15(23):4537. https://doi.org/10.3390/polym15234537
Chicago/Turabian StyleSun, Zebang, Wen Yang, Xiaosa Zhang, Xiaoyu Zhu, Jian Luan, Wenze Li, and Yu Liu. 2023. "Preparation of Novel Nitrogen-Rich Fluorinated Hyperbranched Poly(amide-imide) and Evaluation of Its Electrochromic Properties and Iodine Adsorption Behavior" Polymers 15, no. 23: 4537. https://doi.org/10.3390/polym15234537