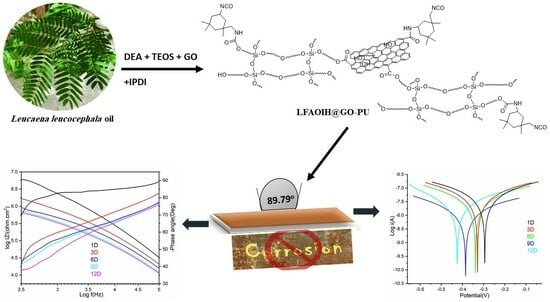
Advanced Anticorrosive Graphene Oxide-Doped Organic-Inorganic Hybrid Nanocomposite Coating Derived from Leucaena leucocephala Oil
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of Leucaena leucocephala Fattyamide Diol (LLFAD)
2.3. Synthesis of LFAOIH and Its PU (LFAOIH-PU30-40)
2.4. Synthesis of LFAOIH@GO and Their PU Nanocomposite (LFAOIH@GOx-PU35)
2.5. Characterizations
3. Results and Discussion
3.1. Spectral Analysis
3.1.1. FTIR Analysis
3.1.2. NMR Analysis
3.1.3. XPS Analysis
3.1.4. Physicomechanical Properties
3.1.5. XRD
3.1.6. Thermal Analysis
3.1.7. Contact Angle
3.1.8. Morphology
3.1.9. Anticorrosion Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhardwaj, N.; Sharma, P.; Kumar, V. Phytochemicals as Steel Corrosion Inhibitor: An Insight into Mechanism. Corros. Rev. 2021, 39, 27–41. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Types of Corrosive Environments. In Corrosion: Understanding the Basics; ASM International: New York, NY, USA, 2000; pp. 193–236.
- Available online: https://www.marketsandmarkets.com/Market-Reports/corrosion-protection-coating-market-150815310.html (accessed on 18 October 2023).
- Ahmad, S.; Zafar, F.; Sharmin, E.; Garg, N.; Kashif, M. Synthesis and Characterization of Corrosion Protective Polyurethanefattyamide/Silica Hybrid Coating Material. Prog. Org. Coat. 2012, 73, 112–117. [Google Scholar] [CrossRef]
- Hammer, P.; Uvida, M.C.; Trentin, A. Self-Healing Organic-Inorganic Coatings. Coatings 2022, 12, 1668. [Google Scholar] [CrossRef]
- Vijayan, J.G.; Chandrashekar, A.; AG, J.; Prabhu, T.N.; Kalappa, P. Polyurethane and Its Composites Derived from Bio-Sources: Synthesis, Characterization and Adsorption Studies. Polym. Polym. Compos. 2022, 30, 09673911221110347. [Google Scholar] [CrossRef]
- Siyanbola, T.O.; Adebowale, A.D.; Adeboye, S.A.; Rao, S.J.V.; Ndukwe, N.A.; Sodiya, E.F.; Ajayi, A.A.; Akintayo, E.T.; Basak, P.; Narayan, R. Development of Functional Polyurethane-Cenosphere Hybrid Composite Coatings from Ricinus Communis Seed Oil. Sci. Afr. 2023, 20, e01711. [Google Scholar] [CrossRef]
- Shaily; Shahzaib, A.; Zafar, F.; Khan, S.; Kaur, B.; Ghosal, A.; Alam, M.; Azam, M.; Haq, Q.M.R.; Nishat, N. Superhydrophobic Mn(II)-Coordinated Technical Cashew Nut Shell Liquid-Based Bactericidal and Corrosion-Resistant Advanced Polyurethane Coatings. Mater. Today Commun. 2023, 35, 105947. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Polyurethanes from Seed Oil-Based Polyols: A Review of Synthesis, Mechanical and Thermal Properties. Ind. Crops Prod. 2019, 142, 111841. [Google Scholar] [CrossRef]
- He, S.; Wei, G.; Zhang, Z.; Yang, L.; Lin, Y.; Du, L.; Du, X. Incorporation of Graphene Oxide Modified with Polyamide Curing Agent into the Epoxy–Zinc Composite Coating for Promoting Its Corrosion Resistance. Polymers 2023, 15, 1873. [Google Scholar] [CrossRef]
- Alrashed, M.M.; Soucek, M.D.; Jana, S.C. Role of Graphene Oxide and Functionalized Graphene Oxide in Protective Hybrid Coatings. Prog. Org. Coat. 2019, 134, 197–208. [Google Scholar] [CrossRef]
- Harb, S.V.; Pulcinelli, S.H.; Santilli, C.V.; Knowles, K.M.; Hammer, P. A Comparative Study on Graphene Oxide and Carbon Nanotube Reinforcement of PMMA-Siloxane-Silica Anticorrosive Coatings. ACS Appl. Mater. Interfaces 2016, 8, 16339–16350. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Alandis, N.M.; Sharmin, E.; Ahmad, N.; Alrayes, B.F.; Ali, D. Characterization of Leucaena (Leucaena leucephala) Oil by Direct Analysis in Real Time (DART) Ion Source and Gas Chromatography. Grasas Y Aceites 2017, 68, 190. [Google Scholar] [CrossRef]
- Zafar, F.; Zafar, H.; Sharmin, E.; Ashraf, S.M.; Ahmad, S. Studies on Ambient Cured Biobased Mn(II), Co(II) and Cu(II) Containing Metallopolyesteramides. J. Inorg. Organomet. Polym. Mater. 2011, 21, 646–654. [Google Scholar] [CrossRef]
- Alam, M.; Alandis, N.M.; Sharmin, E.; Ahmad, N.; Husain, F.M.; Khan, A. Mechanically Strong, Hydrophobic, Antimicrobial, and Corrosion Protective Polyesteramide Nanocomposite Coatings from Leucaena Leucocephala Oil: A Sustainable Resource. ACS Omega 2020, 5, 30383–30394. [Google Scholar] [CrossRef] [PubMed]
- Naebe, M.; Wang, J.; Amini, A.; Khayyam, H.; Hameed, N.; Li, L.H.; Chen, Y.; Fox, B. Mechanical Property and Structure of Covalent Functionalised Graphene/Epoxy Nanocomposites. Sci. Rep. 2014, 4, 4375. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Cook, A.G.; Abberley, J.P.; Walker, R.; Storey, J.M.D.; Imrie, C.T. An FT-IR Spectroscopic Study of the Role of Hydrogen Bonding in the Formation of Liquid Crystallinity for Mixtures Containing Bipyridines and 4-Pentoxybenzoic Acid. RSC Adv. 2016, 6, 108164–108179. [Google Scholar] [CrossRef]
- Huang, X.; Nakagawa, S.; Houjou, H.; Yoshie, N. Insights into the Role of Hydrogen Bonds on the Mechanical Properties of Polymer Networks. Macromolecules 2021, 54, 4070–4080. [Google Scholar] [CrossRef]
- Li, W.; Ma, J.; Wu, S.; Zhang, J.; Cheng, J. The Effect of Hydrogen Bond on the Thermal and Mechanical Properties of Furan Epoxy Resins: Molecular Dynamics Simulation Study. Polym. Test. 2021, 101, 107275. [Google Scholar] [CrossRef]
- Al-Otaibi, W.; Alandis, N.M.; Alam, M. Leucaena Leucocephala Oil-Based Poly Malate-Amide Nanocomposite Coating Material for Anticorrosive Applications. e-Polymers 2023, 23, 20230036. [Google Scholar] [CrossRef]
- Alam, M.; Alandis, N.M.; Zafar, F.; Ghosal, A.; Ahmed, M. Linseed Oil Derived Terpolymer/Silica Nanocomposite Materials for Anticorrosive Coatings. Polym. Eng. Sci. 2021, 61, 2243–2256. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ahmadi, A.; Mahdavian, M. Enhancement of the Corrosion Protection Performance and Cathodic Delamination Resistance of Epoxy Coating through Treatment of Steel Substrate by a Novel Nanometric Sol-Gel Based Silane Composite Film Filled with Functionalized Graphene Oxide Nanosheets. Corros. Sci. 2016, 109, 182–205. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, R.Z.; Ma, Y.Q.; Guan, W.B.; Wu, X.L.; Liu, X.; Li, H.; Du, Y.L.; Pan, C.P. Preparation of Cellulose/Graphene Composite and Its Applications for Triazine Pesticides Adsorption from Water. ACS Sustain. Chem. Eng. 2015, 3, 396–405. [Google Scholar] [CrossRef]
- Ghosh, T.; Karak, N. Mechanically Robust Hydrophobic Interpenetrating Polymer Network-Based Nanocomposite of Hyperbranched Polyurethane and Polystyrene as an Effective Anticorrosive Coating. New J. Chem. 2020, 44, 5980–5994. [Google Scholar] [CrossRef]
- Liang, G.; Yao, F.; Qi, Y.; Gong, R.; Li, R.; Liu, B.; Zhao, Y.; Lian, C.; Li, L.; Dong, X.; et al. Improvement of Mechanical Properties and Solvent Resistance of Polyurethane Coating by Chemical Grafting of Graphene Oxide. Polymers 2023, 15, 882. [Google Scholar] [CrossRef]
- Jiao, X.; Qiu, Y.; Zhang, L.; Zhang, X. Comparison of the Characteristic Properties of Reduced Graphene Oxides Synthesized from Natural Graphites with Different Graphitization Degrees. RSC Adv. 2017, 7, 52337–52344. [Google Scholar] [CrossRef]
- Ashok Kumar, S.S.; Bashir, S.; Ramesh, K.; Ramesh, S. A Comprehensive Review: Super Hydrophobic Graphene Nanocomposite Coatings for Underwater and Wet Applications to Enhance Corrosion Resistance. FlatChem 2022, 31, 100326. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Shenashen, M.A.; El-Sockary, M.A.; Elenien, O.M.A.; EL-Saeed, A.M. Robust Alkyd/Exfoliated Graphene Oxide Nanocomposite as a Surface Coating. Prog. Org. Coat. 2019, 126, 106–118. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, C.; Zhang, Y.; Xie, Z.; Zhou, J.; Kang, J.; Cao, Y.; Xiang, M. Preparation of Well-Dispersed Graphene Oxide-Silica Nanohybrids/Poly(Lactic Acid) Composites by Melt Mixing. Polym. Test. 2023, 118, 107912. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Hao, J.; Zou, G.; Zhang, P.; Wang, G.; Ai, Z.; Chen, H.; Ma, H.; Song, D. Corrosion Resistance and Corrosion Interface Characteristics of Cr-Alloyed Rebar Based on Accelerated Corrosion Testing with Impressed Current. J. Mater. Res. Technol. 2023, 22, 2996–3009. [Google Scholar] [CrossRef]
- Nunes, M.S.; Bandeira, R.M.; Figueiredo, F.C.; dos Santos Junior, J.R.; de Matos, J.M.E. Corrosion Protection of Stainless Steel by a New and Low-cost Organic Coating Obtained from Cashew Nutshell Liquid. J. Appl. Polym. Sci. 2023, 140, e53420. [Google Scholar] [CrossRef]













| Properties | LFAOIH-PU35 | LFAOIH@GO0.25-PU35 | LFAOIH@GO0.5-PU35 | LFAOIH@GO0.75-PU35 |
|---|---|---|---|---|
| Scratch hardness (kg) | 2 | 2.4 | 3.0 | 2.8 |
| Impact (lb/inch) 150 | Pass | Pass | Pass | fail |
| Bending (1/8) | pass | pass | pass | pass |
| Pencil hardness | 3H | 4H | 5H | 4H |
| Cross Hatch (%) | 100 | 100 | 100 | 98 |
| Gloss at 60° | 69 | 75 | 79 | 82 |
| Thickness (micron) | 88 | 129 | 135 | 140 |
| LFAOIH-PU35 | ||||||||||
| Time (Day) | OCP (V) | χ2 | Rs (Ω/cm2) | Rc (MΩ/m2) | CPE1 | |||||
| Y0, pMho*sn | n | |||||||||
| 1 | 0.183 | 0.090 | 638 | 110 | 464 | 0.97 | ||||
| 3 | −0.281 | 0.243 | 622 | 58 | 443 | 0.95 | ||||
| 6 | −0.409 | 0.114 | 615 | 25 | 391 | 0.79 | CPE2 | |||
| Rp1 (KΩ/cm2) | Y0, nMho*sn | n1 | Rp2 (KΩ/cm2) | Y0, µMho*sn | n2 | |||||
| 9 | −0.473 | 0.078 | 436 | 23 | 9.96 | 0.80 | 106 | 2.66 | 0.56 | |
| 12 | −0.478 | 0.074 | 425 | 11.1 | 10.2 | 0.79 | 297 | 12.4 | 0.365 | |
| LFAOIH@GO0.5-PU35 | ||||||||||
| Time (Day) | OCP (V) | χ2 | Rs (Ω/cm2) | Rc (MΩ/cm2) | CPE | |||||
| Y0, nMho*sn | n | |||||||||
| 1 | 0.211 | 0.708 | 616 | 53.4 | 3.58 | 0.911 | ||||
| 3 | −0.311 | 0.192 | 572 | 14.5 | 3.43 | 0.801 | ||||
| 6 | −0.325 | 0.157 | 527 | 12.5 | 3.67 | 0.782 | ||||
| 9 | −0.356 | 0.134 | 526 | 1.50 | 2.97 | 0.782 | ||||
| 12 | −0.402 | 0.152 | 504 | 1.36 | 2.80 | 0.781 | ||||
| Immersion Time (Day) | Ecorr (V) | icorr (A/cm2) | Corrosion Rate (mm/Year) | LPR (Ω) | OCP(V) | IE(%) |
|---|---|---|---|---|---|---|
| LFAOIH-PU35 | ||||||
| 1 | −0.314 | 1.282 × 10−8 | 2.430 × 10−4 | 3.895 × 106 | −0.311 | 99.97 |
| 3 | −0.338 | 2.100 × 10−8 | 2.689 × 10−4 | 2.745 × 106 | −0.333 | 99.95 |
| 6 | −0.364 | 2.314 × 10−8 | 2.930 × 10−4 | 2.683 × 106 | −0.355 | 99.94 |
| 9 | −0.440 | 2.521 × 10−8 | 3.091 × 10−4 | 2.304 × 106 | −0.410 | 99.94 |
| 12 | −0.557 | 1.591 × 10−7 | 1.845 × 10−3 | 1.928 × 105 | −0.478 | 99.96 |
| LFAOIH@GO0.5-PU35 | ||||||
| 1 | −0.303 | 2.170 × 10−8 | 2.249 × 10−4 | 3.846 × 106 | −0.300 | 99.95 |
| 3 | −0.328 | 2.780 × 10−8 | 2.521 × 10−4 | 2.501 × 106 | −0.325 | 99.34 |
| 6 | −0.338 | 2.936 × 10−8 | 3.245 × 10−4 | 2.315 × 106 | −0.333 | 99.93 |
| 9 | −0.385 | 2.350 × 10−8 | 5.556 × 10−4 | 2.024 × 106 | −0.382 | 99.94 |
| 12 | −0.424 | 7.016 × 10−7 | 8.153 × 10−3 | 7.909 × 105 | −0.402 | 99.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-otaibi, W.; Alandis, N.M.; Al-Mohammad, Y.M.; Alam, M. Advanced Anticorrosive Graphene Oxide-Doped Organic-Inorganic Hybrid Nanocomposite Coating Derived from Leucaena leucocephala Oil. Polymers 2023, 15, 4390. https://doi.org/10.3390/polym15224390
Al-otaibi W, Alandis NM, Al-Mohammad YM, Alam M. Advanced Anticorrosive Graphene Oxide-Doped Organic-Inorganic Hybrid Nanocomposite Coating Derived from Leucaena leucocephala Oil. Polymers. 2023; 15(22):4390. https://doi.org/10.3390/polym15224390
Chicago/Turabian StyleAl-otaibi, Wejdan, Naser M. Alandis, Yasser M. Al-Mohammad, and Manawwer Alam. 2023. "Advanced Anticorrosive Graphene Oxide-Doped Organic-Inorganic Hybrid Nanocomposite Coating Derived from Leucaena leucocephala Oil" Polymers 15, no. 22: 4390. https://doi.org/10.3390/polym15224390