Release of Tretinoin Solubilized in Microemulsion from Carbopol and Xanthan Gel: In Vitro versus Ex Vivo Permeation Study
Abstract
:1. Introduction
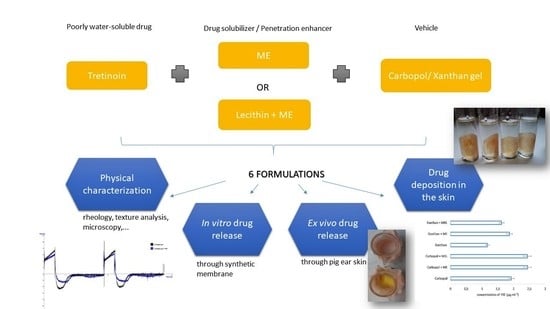
2. Materials and Methods
2.1. Solubility of Tretinoin
2.2. Preparation of Microemulsions
2.3. Preparation of Gels
2.4. Characterization of Gels
2.5. Preparation of Tretinoin Gels
2.6. In Vitro Drug Release
2.7. Ex Vivo Drug Permeation Study
2.8. Drug Release Kinetics
2.9. Drug Deposition in Skin
2.10. Statistical Analysis
3. Results
3.1. Solubility of Tretinoin
3.2. Preparation and Characterization of Gels
3.2.1. Optical Microscopy
3.2.2. Rheology
3.2.3. Texture Analysis
3.3. In Vitro Drug Release
3.4. Ex Vivo Drug Permeation Study
3.5. Drug Release Kinetics
3.6. Drug Deposition in Skin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PubChem Tretinoin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/444795 (accessed on 28 July 2022).
- Raza, K.; Singh, B.; Lohan, S.; Sharma, G.; Negi, P.; Yachha, Y.; Katare, O.P. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int. J. Pharm. 2013, 456, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yoham, A.L.; Casadesus, D. Tretinoin. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK557478/ (accessed on 29 July 2022).
- Rahman, S.A.; Abdelmalak, N.S.; Badawi, A.; Elbayoumy, T.; Sabry, N.; Ramly, A.E. Formulation of tretinoin-loaded topical proniosomes for treatment of acne: In-vitro characterization, skin irritation test and comparative clinical study. Drug Deliv. 2015, 22, 731–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagatin, E.; Wagemaker, T.A.L.; Aguiar Júnior, N.d.R.; Gianeti, M.D.; Gonçalves, E.M.B.; Campos, P.M.B.G.M. Tretinoin-based formulations—influence of concentration and vehicles on skin penetration. Braz. J. Pharm. Sci. 2015, 51, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, S.A.; Pishrochi, S. Formulation and In-vitro Evaluation of Tretinoin Microemulsion as a Potential Carrier for Dermal Drug Delivery. Iran. J. Pharm. Res. 2013, 12, 599. [Google Scholar]
- Darlenski, R.; Surber, C.; Fluhr, J.W. Topical retinoids in the management of photodamaged skin: From theory to evidence-based practical approach. Br. J. Dermatol. 2010, 163, 1157–1165. [Google Scholar] [CrossRef]
- Rahman, S.A.; Abdelmalak, N.S.; Badawi, A.; Elbayoumy, T.; Sabry, N.; El Ramly, A. Tretinoin-loaded liposomal formulations: From lab to comparative clinical study in acne patients. Drug Deliv. 2016, 23, 1184–1193. [Google Scholar] [CrossRef]
- Loureiro, K.D.; Kao, K.K.; Jones, K.L.; Alvarado, S.; Chavez, C.; Dick, L.; Felix, R.; Johnson, D.; Chambers, C.D. Minor malformations characteristic of the retinoic acid embryopathy and other birth outcomes in children of women exposed to topical tretinoin during early pregnancy. Am. J. Med. Genet. A 2005, 136, 117–121. [Google Scholar] [CrossRef]
- Szymański, Ł.; Skopek, R.; Palusińska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kamiński, P.; Zelent, A. Retinoic Acid and Its Derivatives in Skin. Cells 2020, 9, 2660. [Google Scholar] [CrossRef]
- Melhorn, A. Tretinoin. Hautarzt 2017, 68, 941–944. [Google Scholar] [CrossRef]
- Shannon, J.F. Why do humans get acne? A hypothesis. Med. Hypotheses 2020, 134, 109412. [Google Scholar] [CrossRef]
- Schmidt, N.; Gans, E.H. Tretinoin: A Review of Its Anti-inflammatory Properties in the Treatment of Acne. J. Clin. Aesthetic Dermatol. 2011, 4, 22–29. [Google Scholar]
- Ourique, A.F.; Melero, A.; da Silva, C.D.B.; Schaefer, U.F.; Pohlmann, A.R.; Guterres, S.S.; Lehr, C.-M.; Kostka, K.-H.; Beck, R.C.R. Improved photostability and reduced skin permeation of tretinoin: Development of a semisolid nanomedicine. Eur. J. Pharm. Biopharm. 2011, 79, 95–101. [Google Scholar] [CrossRef]
- Ioele, G.; Cione, E.; Risoli, A.; Genchi, G.; Ragno, G. Accelerated photostability study of tretinoin and isotretinoin in liposome formulations. Int. J. Pharm. 2005, 293, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Rosso, J.D.; Harper, J.; Pillai, R.; Moore, R. Tretinoin Photostability. J. Clin. Aesthetic Dermatol. 2013, 6, 25–28. [Google Scholar]
- Morales, J.O.; Valdés, K.; Morales, J.; Oyarzun-Ampuero, F. Lipid nanoparticles for the topical delivery of retinoids and derivatives. Nanomedicine 2015, 10, 253–269. [Google Scholar] [CrossRef] [Green Version]
- Latter, G.; Grice, J.E.; Mohammed, Y.; Roberts, M.S.; Benson, H.A.E. Targeted topical delivery of retinoids in the management of acne vulgaris: Current formulations and novel delivery systems. Pharmaceutics 2019, 11, 490. [Google Scholar] [CrossRef] [Green Version]
- Špaglová, M.; Cuchorova, M.; Čierna, M.; Bauerova, K.; Poništ, S. Microemulsions as Solubilizers and Penetration Enhancers for Minoxidil Release from Gels. Gels 2021, 7, 26. [Google Scholar] [CrossRef]
- Xie, M.; Liu, X.; Wang, S. Degradation of methylene blue through Fenton-like reaction catalyzed by MoS2-doped sodium alginate/Fe hydrogel. Colloids Surf. B Biointerfaces 2022, 214, 112443. [Google Scholar] [CrossRef]
- Arora, R.; Aggarwal, G.; Harikumar, S.L.; Kaur, K. Nanoemulsion Based Hydrogel for Enhanced Transdermal Delivery of Ketoprofen. Adv. Pharm. 2014, 2014, e468456. [Google Scholar] [CrossRef]
- Brisaert, M.; Plaizier-Vercammen, J.A. Investigation on the photostability of tretinoin in creams. Int. J. Pharm. 2007, 334, 56–61. [Google Scholar] [CrossRef]
- Tashtoush, B.M.; Jacobson, E.L.; Jacobson, M.K. UVA is the major contributor to the photodegradation of tretinoin and isotretinoin: Implication for development of improved pharmaceutical formulations. Int. J. Pharm. 2008, 352, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascenso, A.; Vultos, F.; Ferrinho, D.; Salgado, A.; Filho, S.G.; Ferrari, V.; Simões, S.; Marques, H.C. Effect of tretinoin inclusion in dimethyl-beta-cyclodextrins on release rate from a hydrogel formulation. J. Incl. Phenom. Macrocycl. Chem. 2011, 73, 459–465. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.-G.; Liu, X.; Shen, H. Electrospun hierarchical structural films for effective wound healing. Biomater. Adv. 2022, 136, 212795. [Google Scholar] [CrossRef] [PubMed]
- Scomoroscenco, C.; Teodorescu, M.; Raducan, A.; Stan, M.; Voicu, S.N.; Trica, B.; Ninciuleanu, C.M.; Nistor, C.L.; Mihaescu, C.I.; Petcu, C.; et al. Novel Gel Microemulsion as Topical Drug Delivery System for Curcumin in Dermatocosmetics. Pharmaceutics 2021, 13, 505. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Sezer, A.D. Application of Nanotechnology in Drug Delivery; BoD—Books on Demand: Norderstedt, Germany, 2014; ISBN 978-953-51-1628-8. [Google Scholar]
- Franc, A.; Smilková, V.; Rabišková, M.; Vetchý, D.; Kratochvíl, B. Lipofilní formulace pro zvýšení biodostupnosti těžce rozpustných léčivých látek. Chem. Listy 2012, 106, 3–9. Available online: https://www.muni.cz/vyzkum/publikace/1734308 (accessed on 25 July 2022).
- Gibaud, T.; Divoux, T.; Manneville, S. Nonlinear mechanics of colloidal gels: Creep, fatigue and shear-induced yielding. In Statistical and Nonlinear Physics; Springer: New York, NY, USA, 2020; pp. 1–24. Available online: http://arxiv.org/abs/2011.06921 (accessed on 19 July 2022).
- El-Leithy, E.S.; Shaker, D.S.; Ghorab, M.K.; Abdel-Rashid, R.S. Evaluation of Mucoadhesive Hydrogels Loaded with Diclofenac Sodium–Chitosan Microspheres for Rectal Administration. AAPS PharmSciTech 2010, 11, 1695–1702. [Google Scholar] [CrossRef]
- Hurler, J.; Engesland, A.; Poorahmary Kermany, B.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- das Neves, J.; Bahia, M.F. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Potykanowicz, A. Determining the quality of hydrophobic barrier creams by rheological measurements, sensory analysis, pH determination and permeation time measurements. Chemom. Intell. Lab. Syst. 2016, 156, 14–20. [Google Scholar] [CrossRef]
- Blakney, A.K.; Little, A.B.; Jiang, Y.; Woodrow, K.A. In vitro–ex vivo correlations between a cell-laden hydrogel and mucosal tissue for screening composite delivery systems. Drug Deliv. 2017, 24, 582–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honeybrook, A.; Bernstein, E. Oral isotretinoin and photoaging: A review. J. Cosmet. Dermatol. 2020, 19, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Bavarsad, N.; Akhgari, A.; Seifmanesh, S.; Salimi, A.; Rezaie, A. Statistical optimization of tretinoin-loaded penetration-enhancer vesicles (PEV) for topical delivery. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2016, 24, 7. [Google Scholar] [CrossRef] [Green Version]
- Kriangkrai, R.; Chareonvit, S.; Iseki, S.; Limwongse, V. Pretreatment Effect of Folic Acid on 13-Cis-RA-Induced Cellular Damage of Developing Midfacial Processes in Cultured Rat Embryos. Open Dent. J. 2017, 11, 200–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gollnick, H.P.M.; Krautheim, A. Topical treatment in acne: Current status and future aspects. Dermatol. Basel Switz. 2003, 206, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, M.; Samadi, A.; Ahmad Nasrollahi, S.; Farboud, E.S.; Mirrahimi, B.; Hassanzadeh, H.; Nassiri Kashani, M.; Dinarvand, R.; Firooz, A. Tretinoin Loaded Nanoemulsion for Acne Vulgaris: Fabrication, Physicochemical and Clinical Efficacy Assessments. Skin Pharmacol. Physiol. 2018, 31, 316–323. [Google Scholar] [CrossRef]
- Chen, L.; Tan, F.; Wang, J.; Liu, F. Microemulsion: A novel transdermal delivery system to facilitate skin penetration of indomethacin. Pharmazie 2012, 67, 319–323. [Google Scholar] [PubMed]
- Güngör, S.; Bergişadi, N. Effect of penetration enhancers on in vitro percutaneous penetration of nimesulide through rat skin. Pharmazie 2004, 59, 39–41. [Google Scholar]
- Baroli, B.; López-Quintela, M.A.; Delgado-Charro, M.B.; Fadda, A.M.; Blanco-Méndez, J. Microemulsions for topical delivery of 8-methoxsalen. J. Control. Release 2000, 69, 209–218. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Gutierrez-Mendez, N.; Chávez, D.; Leal-Ramos, M. Lecithins: A comprehensive review of their properties and their use in formulating microemulsions. J. Food Biochem. 2022, 46, e14157. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, K.; Araki, M.; Sadaghiani, A.; Khan, A.; Lindman, B. Lecithin-Based Microemulsions: Phase Behavior and Microstructure. Available online: https://pubs.acs.org/doi/pdf/10.1021/j100155a091 (accessed on 8 August 2022).
- Schuh, R.; Bruxel, F.; Teixeira, H. Physicochemical properties of lecithin-based nanoemulsions obtained by spontaneous emulsification or high-pressure homogenization. Quím. Nova 2014, 37, 1193–1198. [Google Scholar] [CrossRef]
- Hwang, S.R.; Lim, S.-J.; Park, J.-S.; Kim, C.-K. Phospholipid-based microemulsion formulation of all-trans-retinoic acid for parenteral administration. Int. J. Pharm. 2004, 276, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.A.; Date, A.A.; Joshi, M.D.; Patravale, V.B. Solid lipid nanoparticles (SLN) of tretinoin: Potential in topical delivery. Int. J. Pharm. 2007, 345, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ghate, V.M.; Lewis, S.A.; Prabhu, P.; Dubey, A.; Patel, N. Nanostructured lipid carriers for the topical delivery of tretinoin. Eur. J. Pharm. Biopharm. 2016, 108, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.; Jiang, Y.; Nakata, T.; Akaki, J.; Matsuoka, N.; Banga, A. Formulation Development and Characterization of Nanoemulsion-Based Formulation for Topical Delivery of Heparinoid. J. Pharm. Sci. 2018, 107, 2883–2890. [Google Scholar] [CrossRef]
- Gupta, S.; Moulik, S. Biocompatible Microemulsions and Their Prospective Uses in Drug Delivery. J. Pharm. Sci. 2008, 97, 22–45. [Google Scholar] [CrossRef]
- Berkó, S.; Balázs, B.; Sütő, B.; Eros, G.; Gál, B.; Sztojkov-Ivanov, A.; Soica, C.; Szabó-Révész, P.; Kemény, L.; Zupko, I.; et al. Monitoring of skin penetration and absorption with a new in vivo experimental model. Farmacia 2014, 62, 1157–1163. [Google Scholar]
- Koutsoulas, C.; Pippa, N.; Demetzos, C.; Zabka, M. Preparation of Liposomal Nanoparticles Incorporating Terbinafine In Vitro Drug Release Studies. J. Nanosci. Nanotechnol. 2014, 14, 4529–4533. [Google Scholar] [CrossRef]
- Koutsoulas, C.; Suleiman, E.; Wagner, A.; Zabka, M. Comparative study between synthetic and phospholipids of natural origin: Effect of phospholipid selection on the behavior of a topical liposomal dosage form incorporating terbinafine. J. Liposome Res. 2014, 24, 336–343. [Google Scholar] [CrossRef]
- Moghimi, H.; Zarghi, A.; Noorani, N. Stereoselective Permeation of Tretinoin and Isotretinoin through Enhancer-Treated Rat Skin.I. Effect of Ethanol and Sodium Dodecyl Sulfate. Iran. J. Pharm. Res. 2003, 2, 127–133. [Google Scholar] [CrossRef]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. In Vitro 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Keck, C.M.; Abdelkader, A.; Pelikh, O.; Wiemann, S.; Kaushik, V.; Specht, D.; Eckert, R.W.; Alnemari, R.M.; Dietrich, H.; Brüßler, J. Assessing the Dermal Penetration Efficacy of Chemical Compounds with the Ex-Vivo Porcine Ear Model. Pharmaceutics 2022, 14, 678. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, V.; Ferková, J.; Žigrayová, D.; Krchňák, D.; Mikuš, P. Comparative Study of Polysaccharide-Based Hydrogels: Rheological and Texture Properties and Ibuprofen Release. Gels 2022, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Moorthy, N.S.H.N.; Maiti, S. Chapter 11—Xanthan-based nanomaterials for drug delivery applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Bera, H., Hossain, C.M., Saha, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 275–292. ISBN 978-0-12-820874-8. Available online: https://www.sciencedirect.com/science/article/pii/B9780128208748000142 (accessed on 12 August 2022).








| ME (w/w) | MEL (w/w) | Carbopol Gel (w/w) | Xanthan Gel (w/w) | |
|---|---|---|---|---|
| Tween 80 | 26.50 | 26.50 | - | - |
| Isopropyl alcohol | 26.50 | 26.50 | - | - |
| Isopropyl myristate | 16.00 | 15.25 | - | - |
| Lecithin 1 | - | 0.75 | - | - |
| Carbopol | - | - | 1.00 | - |
| Xanthan gum | - | - | - | 1.50 |
| NaOH 2 | - | - | 4.00 | - |
| Distilled water | 31.00 | 31.00 | 95.00 | 98.50 |
| Solvent | A | Dilution | TRE Concentration (µg·mL−1) |
|---|---|---|---|
| Isopropyl alcohol | 0.107 ± 0.012 | 1: 2500 | 2400.66 ± 248.63 |
| Tween 80 | 0.071 ± 0.010 | 1: 1000 | 662.25 ± 82.78 |
| Water | 0.194 ± 0.010 | 1: 50 | 84.02 ± 4.14 |
| ME | 0.857 ± 0.006 | 1: 500 | 3584.43 ± 26.61 |
| MEL | 0.848 ± 0.005 | 1: 500 | 3547.19 ± 21.24 |
| Gel | N | |
|---|---|---|
| + | − | |
| Carbopol | 1.325 | 1.140 |
| Carbopol + ME | 1.208 | 1.246 |
| Xanthan | 1.138 | 1.186 |
| Xanthan + ME | 1.202 | 1.154 |
| In Vitro | Ex Vivo | |||||
|---|---|---|---|---|---|---|
| JSS (µg cm−2 h−1) | ER | CP (10−4) | JSS (µg cm−2 h−1) | ER | CP (10−4) | |
| Carbopol | 9.15 | - | 7.63 | 2.70 | - | 2.25 |
| Carbopol + ME | 14.93 | 1.63 | 12.44 | 2.21 | 0.82 | 1.84 |
| Carbopol + MEL | 16.04 | 1.75 | 13.37 | 1.66 | 0.61 | 1.38 |
| Xanthan | 3.49 | - | 2.91 | 1.91 | - | 1.59 |
| Xanthan + ME | 4.20 | 1.20 | 3.50 | 1.67 | 0.87 | 1.39 |
| Xanthan + MEL | 3.43 | 0.98 | 2.86 | 1.52 | 0.80 | 1.27 |
| Carbopol | Carbopol + ME | Carbopol + MEL | Xanthan | Xanthan + ME | Xanthan + MEL | |
|---|---|---|---|---|---|---|
| In Vitro | R2 | R2 | R2 | R2 | R2 | R2 |
| Zero-order | 0.9922 | 0.9839 | 0.9798 | 0.9974 | 0.995 | 0.9934 |
| First-order | 0.9245 | 0.9429 | 0.9439 | 0.9488 | 0.9454 | 0.9382 |
| Higuchi | 0.9587 | 0.9383 | 0.931 | 0.9698 | 0.9624 | 0.9583 |
| Ex Vivo | ||||||
| Zero-order | 0.9987 | 0.9998 | 1.0000 | 0.9999 | 0.9989 | 0.9997 |
| First-order | 0.9357 | 0.9232 | 0.9318 | 0.9458 | 0.9399 | 0.9458 |
| Higuchi | 0.9758 | 0.9858 | 0.9839 | 0.9858 | 0.9902 | 0.9813 |
| A | Ā | S.D. | c (µg·mL−1) | (µg·mL−1) | S.D. | |
|---|---|---|---|---|---|---|
| Carbopol | 0.204 | 0.227 | 0.023 | 1.763 | 1.954 | 0.190 |
| 0.250 | 2.144 | |||||
| 0.227 | 1.954 | |||||
| Carbopol + ME | 0.255 | 0.287 | 0.031 | 2.185 | 2.448 | 0.253 |
| 0.316 | 2.690 | |||||
| 0.289 | 2.467 | |||||
| Carbopol + MEL | 0.253 | 0.286 | 0.046 | 2.169 | 2.442 | 0.377 |
| 0.338 | 2.873 | |||||
| 0.267 | 2.285 | |||||
| Xanthan | 0.101 | 0.131 | 0.043 | 0.911 | 1.159 | 0.354 |
| 0.112 | 1.002 | |||||
| 0.180 | 1.565 | |||||
| Xanthan + ME | 0.226 | 0.217 | 0.009 | 1.945 | 1.871 | 0.075 |
| 0.217 | 0.871 | |||||
| 0.208 | 0.796 | |||||
| Xanthan + MEL | 0.191 | 0.187 | 0.007 | 0.656 | 1.625 | 0.060 |
| 0.179 | 0.556 | |||||
| 0.192 | 0.664 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Špaglová, M.; Papadakos, M.; Čuchorová, M.; Matušová, D. Release of Tretinoin Solubilized in Microemulsion from Carbopol and Xanthan Gel: In Vitro versus Ex Vivo Permeation Study. Polymers 2023, 15, 329. https://doi.org/10.3390/polym15020329
Špaglová M, Papadakos M, Čuchorová M, Matušová D. Release of Tretinoin Solubilized in Microemulsion from Carbopol and Xanthan Gel: In Vitro versus Ex Vivo Permeation Study. Polymers. 2023; 15(2):329. https://doi.org/10.3390/polym15020329
Chicago/Turabian StyleŠpaglová, Miroslava, Martina Papadakos, Mária Čuchorová, and Desana Matušová. 2023. "Release of Tretinoin Solubilized in Microemulsion from Carbopol and Xanthan Gel: In Vitro versus Ex Vivo Permeation Study" Polymers 15, no. 2: 329. https://doi.org/10.3390/polym15020329