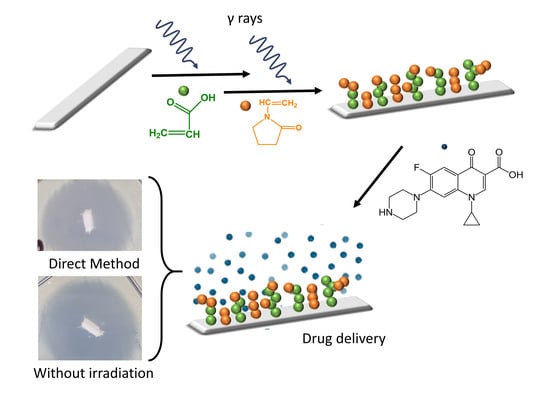
Binary Graft of Poly(acrylic acid) and Poly(vinyl pyrrolidone) onto PDMS Films for Load and Release of Ciprofloxacin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PDMS-g-AAc
2.3. Synthesis of (PDMS-g-AAc)-g-VP
2.4. Infrared Spectroscopy (FTIR-ATR)
2.5. Swelling
2.6. Contact Angle
2.7. Critical pH
2.8. Thermogravimetric Analysis (TGA)
2.9. Loading and Release of Ciprofloxacin for Spectrophotometer UV-Vis
2.9.1. Ciprofloxacin Loading
2.9.2. Ciprofloxacin Release
2.10. Antimicrobials Assay: Agar Diffusion or Kirby-Bauer Method
3. Results
3.1. Synthesis of PDMS-g-AAc
3.2. Synthesis of [PDMS-g-AAc]-g-VP, Second Graft for the Direct Irradiation Method
3.3. Synthesis of [PDMS-g-AAc]-g-V, Second Graft for without Irradiation Method
3.4. Infrared Spectroscopy (FTIR-ATR)
3.5. Swelling
3.6. Contact Angle
3.7. pH Sensitivity
3.8. Thermogravimetric Analysis
3.9. Loading and Release of Ciprofloxacin
3.10. Agar Diffusion or Kirby-Bauer Method
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De, R.; Mahata, M.K.; Kim, K.-T. Structure-Based Varieties of Polymeric Nanocarriers and Influences of Their Physicochemical Properties on Drug Delivery Profiles. Adv. Sci. 2022, 9, 2105373. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Yang, K.; Nie, Z. Engineering heterogeneity of precision nanoparticles for biomedical delivery and therapy. VIEW 2021, 2, 20200067. [Google Scholar] [CrossRef]
- Iyisan, B.; Landfester, K. Polymeric Nanocarriers BT—Biological Responses to Nanoscale Particles: Molecular and Cellular Aspects and Methodological Approaches; Gehr, P., Zellner, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 53–84. ISBN 978-3-030-12461-8. [Google Scholar]
- Haider, M.; Zaki, K.Z.; El Hamshary, M.R.; Hussain, Z.; Orive, G.; Ibrahim, H.O. Polymeric nanocarriers: A promising tool for early diagnosis and efficient treatment of colorectal cancer. J. Adv. Res. 2022, 39, 237–255. [Google Scholar] [CrossRef] [PubMed]
- De Lima, C.S.A.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; Camacho-Cruz, L.A.; Bucio, E.; Kadlubowski, S.S. An updated review of macro, micro, and nanostructured hydrogels for biomedical and pharmaceutical applications. Pharmaceutics 2020, 12, 970. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P. Properties of Polymers. In Polymer Characterization; William Andrew Publishing: Westwood, NJ, USA, 1996; ISBN 978-0-8155-1403-9. [Google Scholar]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and applications of PDMS for biomedical engineering: A Review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Dardouri, M.; Bettencourt, A.; Martin, V.; Carvalho, F.A.; Santos, C.; Monge, N.; Santos, N.C.; Fernandes, M.H.; Gomes, P.S.; Ribeiro, I.A.C. Using plasma-mediated covalent functionalization of rhamnolipids on polydimethylsiloxane towards the antimicrobial improvement of catheter surfaces. Biomater. Adv. 2022, 134, 112563. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, G.; Sinha, S.K.; Chau, F.S.; Wang, S. Lens integrated with self-aligned variable aperture using pneumatic actuation method. Sens. Actuators A Phys. 2010, 159, 105–110. [Google Scholar] [CrossRef]
- Bozukova, D.; Pagnoulle, C.; Jérôme, R.; Jérôme, C. Polymers in modern ophthalmic implants—Historical background and recent advances. Mater. Sci. Eng. R Reports 2010, 69, 63–83. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar Sahani, A. Role of superhydrophobic coatings in biomedical applications. Mater. Today Proc. 2021, 45, 5655–5659. [Google Scholar] [CrossRef]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef]
- Tan, S.H.; Nguyen, N.T.; Chua, Y.C.; Kang, T.G. Oxygen plasma treatment for reducing hydrophobicity of a sealed polydimethylsiloxane microchannel. Biomicrofluidics 2010, 4, 032204. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.H.; Urban, J.P.G.; Cui, Z.; Cui, Z.F. Development of PDMS microbioreactor with well-defined and homogenous culture environment for chondrocyte 3-D culture. Biomed. Microdevices 2006, 8, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Fuentes, Y.S.; Bucio, E.; Burillo, G. Thermo and pH sensitive copolymer based on acrylic acid and N-isopropylacrylamide grafted onto polypropylene. Polym. Bull. 2008, 60, 79–87. [Google Scholar] [CrossRef]
- Cabana, S.; Lecona-Vargas, C.S.; Meléndez-Ortiz, H.I.; Contreras-García, A.; Barbosa, S.; Taboada, P.; Magariños, B.; Bucio, E.; Concheiro, A.; Alvarez-Lorenzo, C. Silicone rubber films functionalized with poly(acrylic acid) nanobrushes for immobilization of gold nanoparticles and photothermal therapy. J. Drug Deliv. Sci. Technol. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalt. Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, T.; Yoshioka, S.; Aso, Y.; Kojima, S. Ability of polyvinylpyrrolidone and polyacrylic acid to inhibit the crystallization of amorphous acetaminophen. J. Pharm. Sci. 2004, 93, 2710–2717. [Google Scholar] [CrossRef]
- Kadłubowski, S.; Henke, A.; Ulański, P.; Rosiak, J.M.; Bromberg, L.; Hatton, T.A. Hydrogels of polyvinylpyrrolidone (PVP) and poly(acrylic acid) (PAA) synthesized by photoinduced crosslinking of homopolymers. Polymer 2007, 48, 4974–4981. [Google Scholar] [CrossRef]
- Rizwan, M.; Rubina Gilani, S.; Iqbal Durani, A.; Naseem, S. Materials diversity of hydrogel: Synthesis, polymerization process and soil conditioning properties in agricultural field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef]
- Ito, T.; Yamaguchi, S.; Soga, D.; Yoshimoto, T.; Koyama, Y. Preparation of a bioadhesive Poly(Acrylic Acid)/Polyvinylpyrrolidone complex gel and its clinical effect on dental hemostasis. Gels 2022, 8, 462. [Google Scholar] [CrossRef] [PubMed]
- Bucio, E.; Burillo, G. Radiation-induced grafting of sensitive polymers. J. Radioanal. Nucl. Chem. 2009, 280, 239–243. [Google Scholar] [CrossRef]
- Velazco-Medel, M.A.; Camacho-Cruz, L.A.; Magaña, H.; Palomino, K.; Bucio, E. Simultaneous grafting polymerization of acrylic acid and silver sggregates formation by direct reduction using γ radiation onto silicone surface and their antimicrobial activity and biocompatibility. Molecules 2021, 26, 2859. [Google Scholar] [CrossRef]
- Feairheller, W.R.; Katon, J.E. The vibrational spectra of acrylic acid and sodium acrylate. Spectrochim. Acta Part A Mol. Spectrosc. 1967, 23, 2225–2232. [Google Scholar] [CrossRef]
- Casimiro, M.; Gomes, S.; Rodrigues, G.; Leal, J.; Ferreira, L. Chitosan/Poly(vinylpyrrolidone) Matrices Obtained by Gamma-Irradiation for Skin Scaffolds: Characterization and Preliminary Cell Response Studies. Materials 2018, 11, 2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Faria, P.; Martin, A.; Alves, N. Caracterização no Infravermelho (IV) e Eletrônica de superfície (MEV) de membranas assimétricas à base de Poli (acrilonitrila-co-acetato de vinila). Matéria 2017, 22. [Google Scholar] [CrossRef] [Green Version]
- Haaf, F.; Sanner, A.; Straud, F. Polymers of N-Vinylpyrrolidone: Synthesis, characterization and uses. Polym. J. 1985, 17, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Gokaltun, A.; Yarmush, M.L.; Asatekin, A.; Usta, O.B. Recent advances in nonbiofouling PDMS surface modification strategies applicable to microfluidic technology. Technology 2017, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.P.; Shukla, T.; Dhote, V.K.; Mishra, D.K.; Maheshwari, R.; Tekade, R.K. Use of Polymers in Controlled Release of Active Agents; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128179093. [Google Scholar]
- Chun, M.-K.; Cho, C.-S.; Choi, H.-K. Characteristics of poly(vinyl pyrrolidone)/poly(acrylic acid) interpolymer complex prepared by template polymerization of acrylic acid: Effect of reaction solvent and molecular weight of template. J. Appl. Polym. Sci. 2004, 94, 2390–2394. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- CLSI Document M100-S21; Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011; ISBN 1-56238-742-1.
- Tascini, C.; Sozio, E.; Viaggi, B.; Meini, S. Reading and understanding an antibiogram. Ital. J. Med. 2016, 10, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Palsule, A.; Clarson, S.; Widenhouse, C. Gamma Irradiation of Silicones. J. Inorg. Organomet. Polym. Mater. 2008, 18, 207–221. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Park, J.-S.; Kim, E.J.; Jeong, S.-I.; Lee, J.Y.; Lim, Y.-M. Preparation of Radiation Cross-Linked Poly(Acrylic Acid) Hydrogel Containing Metronidazole with Enhanced Antibacterial Activity. Int. J. Mol. Sci. 2019, 21, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, C.; Mi, Y. A study of blending and complexation of poly(acrylic acid)/poly(vinyl pyrrolidone). Polymer 2002, 43, 823–829. [Google Scholar] [CrossRef]












| Sample | k1 | k2 | M | Tlag |
|---|---|---|---|---|
| PDMS-g-AAc (3%) | 44.7 ± 3.9 | −14.4 ± 2.3 | 0.28 ± 0.02 | 0.29 ± 0.09 |
| [PDMS-g-AAc (3%)]-g-VP (10%) DM | 133.8 ± 14.3 | −54.1 ± 4.6 | 0.14 ± 0.01 | 0.49 ± 0.01 |
| [PDMS-g-AAc (3%)]-g-VP (10%) WR | 39.5 ± 2.6 | −10.8 ± 2.4 | 0.30 ± 0.07 | xx |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santillán-González, B.; Duarte-Peña, L.; Bucio, E. Binary Graft of Poly(acrylic acid) and Poly(vinyl pyrrolidone) onto PDMS Films for Load and Release of Ciprofloxacin. Polymers 2023, 15, 302. https://doi.org/10.3390/polym15020302
Santillán-González B, Duarte-Peña L, Bucio E. Binary Graft of Poly(acrylic acid) and Poly(vinyl pyrrolidone) onto PDMS Films for Load and Release of Ciprofloxacin. Polymers. 2023; 15(2):302. https://doi.org/10.3390/polym15020302
Chicago/Turabian StyleSantillán-González, Belén, Lorena Duarte-Peña, and Emilio Bucio. 2023. "Binary Graft of Poly(acrylic acid) and Poly(vinyl pyrrolidone) onto PDMS Films for Load and Release of Ciprofloxacin" Polymers 15, no. 2: 302. https://doi.org/10.3390/polym15020302