Synthesis of Multifunctional Oligomethylsilsesquioxanes by Catalyst-Free Hydrolytic Polycondensation of Methyltrimethoxysilane under Microwave Radiation
Abstract
:1. Introduction
2. Materials and Methods
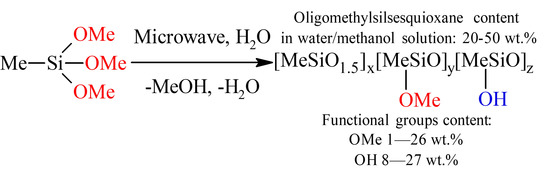
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khananashvili, L.M.; Andrianov, K.A. Tekhnologiya Elementorganicheskikh Monomerov i Polimerov (Technology of Organometallic Monomers and Polymers); Khimiya: Moscow, Russia, 1983. [Google Scholar]
- Baney, R.H.; Iton, M.; Sakakabara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Egorova, E.V.; Vasilenko, N.G.; Demchenko, N.V.; Tatarinova, E.A.; Muzafarov, A.M. Polycondensation in an active medium—universal method for producing polyorganosiloxanes. Dokl. Chem. (Engl. Transl.) 2009, 424, 15–19. [Google Scholar] [CrossRef]
- Meshkov, I.B.; Kalinina, A.A.; Kazakova, V.V.; Demchenko, A.I. Densely Cross-Linked Polysilixant Nanogeis. INEOS OPEN 2020, 3, 118–132. [Google Scholar] [CrossRef]
- Grotex Ltd. Polymethylsiloxane Adsorbent Polyhydrate and Method for Its Production. RU Patent 2761627 C1, 1 December 2020. [Google Scholar]
- Muzafarov, A.M. Efficient Methods for Preparing Silicon Compounds; Roesky, H.W., Ed.; Academic Press: London, UK, 2016; pp. 179–181. [Google Scholar]
- Kumar, V.; Alam, M.N.; Manikkavel, A.; Song, M.; Lee, D.J.; Park, S.S. Silicone Rubber Composites Reinforced by Carbon Nanofillers and Their Hybrids for Various Applications: A Review. Polymers 2021, 13, 2322. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, K.; Nakanishi, K. Controlled pore formation in organotrialkoxysilane-derived hybrids: From aerogels to hierarchically porous monoliths. Chem. Soc. Rev. 2011, 40, 754–770. [Google Scholar] [CrossRef]
- Laird, M.; Gaveau, P.; Trens, P.; Carcel, C.; Unno, M.; Bartlett, J.R.; Man, M.W.C. Post-synthesis modification of functionalised polyhedral oligomeric silsesquioxanes with encapsulated fluoride—enhancing reactivity of T 8 -F POSS for materials synthesis. New J. Chem. 2021, 45, 4227–4235. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Advanced functional materials based on polyhedral oligomeric silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 1733–1746. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Yang, G.; Wang, F.; Nie, J.; Ma, C.; Gao, M. POSS hybrid hydrogels: A brief review of synthesis, properties and applications. Eur. Polym. J. 2021, 143, 110–180. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.; Janowski, D.; Pielichowski, J. Polyhedral Oligomeric Silsesquioxanes (POSS)—Containing Nanohybrid Polymers. Adv. Polym. Sci. 2006, 201, 225–296. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, G.; Zhang, C. Promoted ablation resistance of polydimethylsiloxane via crosslinking with multi-ethoxy POSS. Eng. Mater. Sci. Compos. Part B-Eng. 2020, 190, 107901. [Google Scholar] [CrossRef]
- Obrezkova, M.A.; Kalinina, A.A.; Pavlichenko, I.V.; Vasilenko, N.G.; Mironova, M.V.; Semakov, A.V.; Kulichikhin, V.G.; Buzin, M.I.; Muzafarov, A.M. Comb-Like Polymethylsiloxanes. Synthesis, Structure and Properties. Silicon 2015, 7, 177–189. [Google Scholar] [CrossRef]
- Vysochinskaya, Y.S.; Gorodov, V.V.; Anisimov, A.A.; Boldyrev, K.L.; Buzin, M.I.; Naumkin, A.V.; Maslakov, K.I.; Peregudov, A.S.; Shchegolikhina, O.I.; Muzafarov, A.M. New star-like polydimethylsiloxanes: Synthesis, properties, and application. Russ. Chem. Bull 2017, 66, 10941–11098. [Google Scholar] [CrossRef]
- Migulin, D.; Tatarinova, E.; Meshkov, I.; Cherkaev, G.; Vasilenko, N.; Buzin, M.; Muzafarov, A. Synthesis of the first hyperbranched polyorganoethoxysilsesquioxanes and their chemical transformations to functional core–shell nanogel systems. Polym. Int. 2016, 65, 72–83. [Google Scholar] [CrossRef]
- Shchegolikhina, O.I.; Anisimov, A.A.; Shchemelinina, M.V.; Zhemchugov, P.V.; Goloveshkin, A.S.; Korlukov, A.A.; Kononova, E.G.; Pigaleva, M.A.; Elmanovich, I.V.; Gallimov, M.O.; et al. Synthesis of Macrocyclic Siloxane Polyol in Carbonic Acid. Macroheterocycles 2015, 58, 193–198. [Google Scholar] [CrossRef]
- Anisimov, A.A.; Polshchikova, N.V.; Vysochinskaya, Y.S.; Zader, P.A.; Nikiforova, G.G.; Peregudov, A.S.; Buzin, M.I.; Shchegolikhina, O.I.; Muzafarov, A.M. Condensation of all-cis-tetraphenylcyclotetrasiloxanetetraol in ammonia: New method for preparation of ladder-like polyphenylsilsesquioxanes. Mendeleev. Commun. 2019, 29, 421–423. [Google Scholar] [CrossRef]
- Temnikov, M.N.; Zimovets, S.N.; Vasil’ev, V.G.; Buzin, M.I. Polyphenylsesquioxane Nanogels as Regulators of the Mechanical Properties of Vulcanizates on PDMS. INEOS OPEN 2020, 3, 112–117. [Google Scholar] [CrossRef]
- Brown, J.F., Jr.; Vogt, L.H., Jr.; Katchman, A.; Eustance, J.W.; Kiser, K.M.; Krantz, K.W. Double chain polymers of phenylsilsesquioxane. J. Am. Chem. Soc. 1960, 82, 6194–6195. [Google Scholar] [CrossRef]
- Harreld, J.H.; Su, K.; Katsoulis, D.E.; Suto, M.; Stucky, G.D. Surfactant and pH-Mediated Control over the Molecular Structure of Poly(phenylsilsesquioxane) Resins. Chem. Mater. 2002, 14, 1174–1182. [Google Scholar] [CrossRef]
- Chernyshev, E.A.; Talanov, V.N. Himiya Elementoorganicheskih Monomerov i Polimerov (Chemistry of Organoelement Monomers and Polymers); KolosS: Moscow, Russia, 2011. [Google Scholar]
- Ivanov, P.V.; Mazhorova, N.G. Comparative analysis of phase diagrams of organochlorosilane/organoalkoxysilane—Solvent—Water systems. Russ. Chem. Bull 2020, 69, 1061–1071. [Google Scholar] [CrossRef]
- Ivanov, A.G.; Kopylov, V.M.; Kireev, V.V.; Borisov, R.S.; Fedotova, T.I.; Bilichenko, Y.V. A MALDI mass spectrometry investigation of the compositions of the products of the partial acidolysis of MeSi (OMe)3. Polym. Sci. Ser. 2014, 56, 49–54. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okhapkin, I.M.; Makhaeva, E.E.; Khokhlov, A.R. Water Solutions of Amphiphilic Polymers: Nanostructure Formation and Possibilities for Catalysis. Adv. Polym. Sci. 2006, 195, 177–210. [Google Scholar] [CrossRef]
- Kalinina, A.A.; Kholodkov, D.N.; Meshkov, I.B.; Pigaleva, M.A.; Elmanovich, I.V.; Molodtsova, Y.A.; Gallyamov, M.O.; Muzafarov, A.M. Hydrolytic polycondensation of methyltrialkoxysilanes under pressure. Russ. Chem. Bull 2016, 65, 1104–1109. [Google Scholar] [CrossRef]
- Kalinina, A.A.; Zhiltsov, A.S.; Pigaleva, M.A.; Elmanovich, I.V.; Molodtsova, Y.A.; Kotov, V.M.; Muzafarov, A.M. Non-catalytic hydrolytic polycondensation of dialkoxydiorganosilanes under elevated pressure. Russ. Chem. Bull. 2017, 66, 355–361. [Google Scholar] [CrossRef]
- Kalinina, A.A.; Elmanovich, I.V.; Temnikov, M.N.; Pigaleva, M.A.; Zhiltsov, A.S.; Gallyamov, M.O.; Muzafarov, A.M. Hydrolytic polycondensation of diethoxydimethylsilane in carbonic acid. RSC Adv. 2014, 5, 5664–5666. [Google Scholar] [CrossRef]
- Kalinina, A.A.; Pryakhina, T.A.; Talalaeva, E.V.; Vasilenko, N.G.; Pigaleva, M.A.; Elmanovich, I.V.; Muzafarov, A.M. Hydrolytic polycondensation of diethoxymethylsilane under pressure. Russ. Chem. Bull. 2022, 71, 1648–1655. [Google Scholar] [CrossRef]
- Yakhontov, N.G.; Gorbatsevich, O.B.; Kalinina, A.A.; Demchenko, N.V.; Kazakova, V.V.; Muzafarov, A.M. Hydrolytic polycondensation of trimethoxymethylsilane under ultrasonic irradiation. Mendeleev Commun. 2020, 30, 336–338. [Google Scholar] [CrossRef]
- Ogawa, T.; Watanabe, J.; Oshima, Y. Catalyst-free synthesis of polyorganosiloxanes by high temperature and pressure water. II. Understanding of the reaction process. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2656–2663. [Google Scholar] [CrossRef]
- A.N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences. Method for Obtaining Soluble Polymethylsilsesquioxanes. RU Patent 2615507 C1, 11 December 2015. [Google Scholar]
- Stojanovic, A.; Comesaña, S.P.; Rentsch, D.; Koebel, M.M.; Malfait, W.J. Ambient pressure drying of silica aerogels after hydrophobization with mono-, di-and tri-functional silanes and mixtures thereof. Microporous Mesoporous Mater. 2019, 284, 289–295. [Google Scholar] [CrossRef]
- Irfan, M.H. Silicones in the construction industry. In Chemistry and Technology of Thermosetting Polymers in Construction Applications; Springer: Dordrecht, The Netherlands, 1998; pp. 170–202. [Google Scholar]
- Lee, L.H.; Chen, W.C.; Liu, W.C. Structural control of oligomeric methyl silsesquioxane precursors and their thin-film properties. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 1560–1571. [Google Scholar] [CrossRef]
- Iwamura, T.; Adachi, K.; Chujo, Y. Simple and Rapid Eco-friendly Synthesis of Cubic Octamethylsilsesquioxane Using Microwave Irradiation. Chem. Lett. 2010, 39, 354–355. [Google Scholar] [CrossRef]
- Lovingood, D.D.; Owens, J.R.; Seeber, M.; Kornev, K.G.; Luzinovm, I. Preparation of Silica Nanoparticles Through Microwave-assisted Acid-catalysis. J. Vis. Exp. 2013, 82, e51022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Conto, J.F.; Oliveira, M.R.; Oliveira, M.M.; Brandão, T.G.; Campos, K.V.; Santana, C.C.; Egues, S.M. One-pot synthesis and modification of silica nanoparticles with 3-chloropropyltrimethoxysilane assisted by microwave irradiation. Chem. Eng. Commun. 2018, 205, 533–537. [Google Scholar] [CrossRef]
- Lee, A.W.H.; Gates, D.B. Rapid Covalent Modification of Silicon Oxide Surfaces through Microwave-Assisted Reactions with Alcohols. Langmuir 2016, 32, 7284–7293. [Google Scholar] [CrossRef]
- Armaredo, L.F.; Perkin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth Heinemann: Oxford, UK, 1999. [Google Scholar]














| Sample | Reaction Conditions | Product Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reagent Ratio MTMS/H2O, mol/mol | T, °C | Power, W | t, min | MTMS Conversion, % | OMe-Group Conversion, % | Oligomeric Fraction | |||||
| Yield, % | Mp (GPC) | [MeSiO1.5]/[OMe]/[OH] (1H NMR Data) | Content of Groups (wt.%) | ||||||||
| OMe | OH | ||||||||||
| 1 | 1/0.5 | 30 | 20 | 90 | 42 | 13 | 6 | 400 | 1/0.69/0.44 | 25 | 8 |
| 2 | 1/1 | 30 | 20 | 90 | 82 | 48 | 20 | 600 | 1/0.77/0.60 | 26 | 11 |
| 3 | 1/1.5 | 30 | 20 | 90 | 100 | 70 | 75 | 850 | 1/0.68/0.52 | 24 | 10 |
| 4 | 1/3 | 30 | 20 | 90 | 100 | 81 | 79 | 900 | 1/0.23/1.00 | 9 | 19 |
| 5 | 1/6 | 30 | 20 | 90 | 100 | 95 | 84 | 950 | 1/0.06/1.06 | 2 | 21 |
| 6 | 1/9 | 30 | 20 | 90 | 100 | 96 | 75 | 1015 | 1/0.03/1.24 | 1 | 24 |
| 7 | 1/1.5 | 30 | 20 | 5 | 64 | 18 | 29 | 600 | 1/0.25/0.47 | 10 | 10 |
| 8 | 1/1.5 | 30 | 20 | 15 | 80 | 54 | 27 | 600 | 1/0,17/1,38 | 6 | 25 |
| 9 | 1/1.5 | 30 | 20 | 30 | 93 | 52 | 30 | 700 | 1/0.29/1.08 | 11 | 20 |
| 10 | 1/1.5 | 30 | 20 | 60 | 98 | 69 | 67 | 800 | 1/0.74/0.65 | 26 | 12 |
| 11 | 1/3 | 30 | 20 | 2.5 | 44 | 10 | 32 | 900 | 1/0.94/0.83 | 30 | 14 |
| 12 | 1/3 | 30 | 20 | 5 | 96 | 76 | 48 | 940 | 1/0.13/1.21 | 5 | 23 |
| 13 | 1/3 | 30 | 20 | 15 | 97 | 78 | 68 | 915 | 1/0.16/1.09 | 6 | 21 |
| 14 | 1/3 | 30 | 20 | 30 | 98 | 75 | 74 | 930 | 1/0.23/1.40 | 8 | 25 |
| 15 | 1/3 | 30 | 20 | 60 | 100 | 81 | 77 | 920 | 1/0.24/1.27 | 9 | 23 |
| 16 | 1/1.5 | 40 | 20 | 5 | 77 | 62 | 23 | 915 | 1/0.24/1.07 | 9 | 20 |
| 17 | 1/1.5 | 50 | 20 | 5 | 89 | 64 | 38 | 860 | 1/0.23/1.00 | 9 | 19 |
| 18 | 1/1.5 | 30 | 50 | 5 | 95 | 72 | 29 | 870 | 1/0.18/1.25 | 7 | 23 |
| 19 | 1/1.5 | 30 | 100 | 5 | 90 | 63 | 26 | 890 | 1/0.14/1.33 | 5 | 24 |
| 20 | 1/1.5 | 30 | 150 | 5 | 85 | 71 | 21 | 900 | 1/0.10/1.54 | 4 | 27 |
| 21 | 1/1.5 | 30 | 300 | 5 | 81 | 63 | 17 | 975 | 1/0.13/1.21 | 5 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinina, A.A.; Gorbatsevich, O.B.; Yakhontov, N.G.; Demchenko, N.V.; Vasilenko, N.G.; Kazakova, V.V.; Muzafarov, A.M. Synthesis of Multifunctional Oligomethylsilsesquioxanes by Catalyst-Free Hydrolytic Polycondensation of Methyltrimethoxysilane under Microwave Radiation. Polymers 2023, 15, 291. https://doi.org/10.3390/polym15020291
Kalinina AA, Gorbatsevich OB, Yakhontov NG, Demchenko NV, Vasilenko NG, Kazakova VV, Muzafarov AM. Synthesis of Multifunctional Oligomethylsilsesquioxanes by Catalyst-Free Hydrolytic Polycondensation of Methyltrimethoxysilane under Microwave Radiation. Polymers. 2023; 15(2):291. https://doi.org/10.3390/polym15020291
Chicago/Turabian StyleKalinina, Alexandra A., Olga B. Gorbatsevich, Nikita G. Yakhontov, Nina V. Demchenko, Nataliya G. Vasilenko, Valentina V. Kazakova, and Aziz M. Muzafarov. 2023. "Synthesis of Multifunctional Oligomethylsilsesquioxanes by Catalyst-Free Hydrolytic Polycondensation of Methyltrimethoxysilane under Microwave Radiation" Polymers 15, no. 2: 291. https://doi.org/10.3390/polym15020291