Improvement in Phase Compatibility and Mechanical Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/thermoplastic Starch Blends with Citric Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of TPS with CA
2.3. Preparation of PLLA-PEG-PLLA/TPS Blends
2.4. Characterization of Modified TPS and PLLA-PEG-PLLA/TPS Blends
3. Results and Discussion
3.1. FTIR Spectroscopy of the Unmodified and Modified TPS
3.2. FTIR Spectroscopy of the PLLA-PEG-PLLA/TPS Blends
3.3. Thermal Transition Properties of the PLLA-PEG-PLLA/TPS Blends
3.4. Thermal Decomposition Properties of the PLLA-PEG-PLLA/TPS Blends
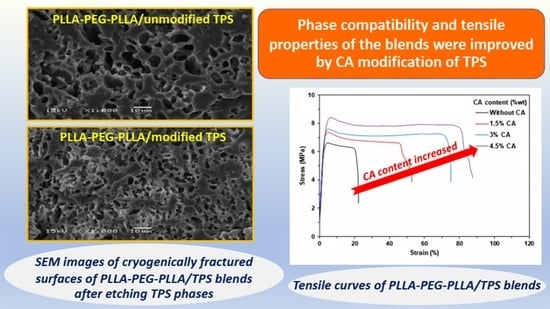
3.5. Phase Compatibility of the PLLA-PEG-PLLA/TPS Blends
3.6. Water Contact Angles of the PLLA-PEG-PLLA/TPS Blends
3.7. Mechanical Properties of the PLLA-PEG-PLLA/TPS Blends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durpekova, S.; Bergerova, E.D.; Hanusova, D.; Dusankova, M.; Sedlarik, V. Eco-friendly whey/polysaccharide-based hydrogel with poly(lactic acid) for improvement of agricultural soil quality and plant growth. Int. J. Biol. Macromol. 2022, 212, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, A.; Cuc, S.; Prodan, D.; Rusu, M.; Popa, D.; Taut, A.C.; Petean, I.; Bombo¸s, D.; Doukeh, R.; Nemes, O. Development and characterization of polylactic acid (PLA)-based nanocomposites used for food packaging. Polymers 2023, 15, 2855. [Google Scholar] [CrossRef] [PubMed]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A comprehensive review on polylactic acid (PLA)–Synthesis, processing and application in food packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Perkins, P.; Yi, R.; Ren, T. Development of polylactic acid based antimicrobial food packaging films with N-halamine modified microcrystalline cellulose. Int. J. Biol. Macromol. 2023, 242 Pt 1, 124685. [Google Scholar]
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable polylactic acid and its composites: Characteristics, processing, and sustainable applications in sports. Polymers 2023, 15, 3096. [Google Scholar] [CrossRef]
- Pérez-Davila, S.; Garrido-Gulías, N.; González-Rodríguez, L.; López-Álvarez, M.; Serra, J.; López-Periago, J.E.; González, P. Physicochemical properties of 3D-printed polylactic acid/hydroxyapatite scaffolds. Polymers 2023, 15, 2849. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, J.; Wu, Y.; Yin, D.; Chen, S.; Tu, H.; Wei, T.; Zhang, C.; Zhu, H.; Jin, H. Highly enhanced mechanical, thermal, and crystallization performance of PLA/PBS composite by glass fiber coupling agent modification. Polymers 2023, 15, 3164. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Blanes-Martinez, M.M.; Montanes, N.; Fenollar, O.; Torres-Giner, S.; Balart, R. Reactive toughening of injection-molded polylactide pieces using maleinixed hemp seed oil. Eur. Polym. J. 2018, 98, 402–410. [Google Scholar] [CrossRef]
- Jin, F.L.; Hu, R.R.; Park, S.J. Improvement of thermal behaviors of biodegradable poly(lactic acid) polymer: A review. Compos. B Eng. 2019, 164, 287–296. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Baimark, Y.; Srihanam, P.; Srisuwan, Y.; Phromsopha, T. Enhancement in crystallizability of poly(L-lactide) using stereocomplex-polylactide powder as a nucleating agent. Polymers 2022, 14, 4092. [Google Scholar] [CrossRef] [PubMed]
- Baimark, Y.; Rungseesantivanon, W.; Prakymorama, N. Improvement in melt flow property and flexibility of poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) by chain extension reaction for potential use as flexible bioplastics. Mater. Des. 2018, 154, 73–80. [Google Scholar] [CrossRef]
- Baimark, Y.; Rungseesantivanon, W.; Prakymoramas, N. Synthesis of flexible poly(L-lactide)-b-polyethylene glycol-b-poly(L-lactide) bioplastics by ring-opening polymerization in the presence of chain extender. e-Polymers 2020, 20, 423–429. [Google Scholar] [CrossRef]
- Thongsomboon, W.; Srihanam, P.; Baimark, Y. Preparation of flexible poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/talcum/thermoplastic starch ternary composites for use as heat-resistant and single-use bioplastics. Int. J. Biol. Macromol. 2023, 230, 123172. [Google Scholar] [CrossRef]
- Li, D.; Luo, C.; Zhou, J.; Dong, L.; Chen, Y.; Liu, G.; Qiao, S. The Rrole of the interface of PLA with thermoplastic starch in the nonisothermal crystallization behavior of PLA in PLA/thermoplastic starch/SiO2 composites. Polymers 2023, 15, 1579. [Google Scholar] [CrossRef]
- Jaouadi, N.; Al-Itry, R.; Maazouz, A.; Lamnawar, K. Biaxial orientation of PLA/PBAT/thermoplastic cereal flour sheets: Structure–processing– property relationships. Polymers 2023, 15, 2068. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Ismail, H. A review on tensile and morphological properties of poly(lactic acid) (PLA)/thermoplastic starch (TPS) blends. Polym-Plast. Tech. Mater. 2019, 58, 1945–1964. [Google Scholar] [CrossRef]
- Srisuwan, Y.; Baimark, Y. Thermal, morphological and mechanical properties of flexible poly(l-lactide)-b-polyethylene glycol-b-poly(l-lactide)/thermoplastic starch blends. Carbohydr. Polym. 2022, 283, 119155. [Google Scholar] [CrossRef]
- Huneault, M.A.; Li, H. Morphology and properties of compatibilized polylactide/thermoplastic starch blends. Polymer 2007, 48, 270–280. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, C.; Ma, S.; Feng, J.; Yang, Y.; Zhang, R.; Zhu, J. The properties of poly(lactic acid)/starch blends with a functionalized plant oil: Tung oil anhydride. Carbohydr. Polym. 2013, 95, 77–84. [Google Scholar] [CrossRef]
- Wang, N.; Yu, J.; Chang, P.R.; Ma, X. Influence of formamide and water on the properties of thermoplastic starch/poly(lactic acid) blends. Carbohyd. Polym. 2008, 71, 109–118. [Google Scholar] [CrossRef]
- Ibrahim, N.; Wahab, M.K.A.; Uylan, D.N.; Ismail, H. Physical and degradation properties of polylactic acid and thermoplastic starch blends–effect of citric acid treatment on starch structures. Bioresources 2017, 12, 3076–3087. [Google Scholar] [CrossRef]
- Kahar, A.W.M.; Ismail, H.; Othman, N. Morphology and tensile properties of high-density polyethylene/natural rubber/thermoplastic tapioca starch blends: The effect of citric acid-modified tapioca starch. J. Appl. Polym. Sci. 2012, 125, 768–775. [Google Scholar] [CrossRef]
- Srihanam, P.; Thongsomboon, W.; Baimark, Y. Phase morphology, mechanical, and thermal properties of calcium carbonate-reinforced poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide) bioplastics. Polymers 2023, 15, 301. [Google Scholar] [CrossRef]
- Carvalho, A.J.F.; Zambon, M.D.; Curvelo, A.A.D.S.; Gandini, A. Thermoplastic starch modification during melt processing: Hydrolysis catalyzed by carboxylic acids. Carbohyd. Polym. 2005, 62, 387–390. [Google Scholar] [CrossRef]
- Ning, W.; Jiugao, Y.; Xiaofei, M.; Ying, W. The influence of citric acid on the properties of thermoplastic starch/linear low-density polyethylene blends. Carbohyd. Polym. 2007, 67, 446–453. [Google Scholar] [CrossRef]
- Cuevas-Carballo, Z.B.; Duarte-Aranda, S.; Canch’ e-Escamilla, G. Properties and biodegradation of thermoplastic starch obtained from grafted starches with poly(lactic acid). J. Polym. Environ. 2019, 27, 2607–2617. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Z.; Liu, Q.; Han, Y.; Zhang, L.; Chen, D.; Tian, W. Characterization of citric acid/glycerol co-plasticized thermoplastic starch prepared by melt blending. Carbohyd. Polym. 2007, 69, 748–755. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J.; Stumborg, M. Properties of biodegradable citric acid-modified granular starch/thermoplastic pea starch composites. Carbohydr. Polym. 2009, 75, 1–8. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Antosik, A.K.; Zdanowicz, M. The effect of citric acid on physicochemical properties of hydrophilic carboxymethyl starch-based films. J. Polym. Environ. 2019, 27, 1379–1387. [Google Scholar] [CrossRef]
- Murillo, E.A. In situ compatibilization of thermoplastic starch/polylactic acid blends using citric acid. Macromol. Res. 2023, 31, 157–169. [Google Scholar] [CrossRef]
- Ferrarezi, M.M.F.; de Oliveira Taipina, M.; Escobar da Silva, L.C.; Goncalves, M.C. Poly(ethylene glycol) as a compatibilizer for poly(lactic acid)/thermoplastic starch blends. J. Polym. Environ. 2013, 21, 151–159. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Raquez, J.-M.; Dubois, P.; Kenny, J.M.; Peponi, L. Thermal and composting degradation of EVA/thermoplastic starch blends and their nanocomposites. Polym. Degrad. Stab. 2019, 159, 184–198. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Vu, T.T.; Grillet, A.-C.; Ha Thuc, H.; Ha Thuc, C.N. Effect of organoclay on morphology and properties of linear low density polyethylene and Vietnamese cassava starch biobased blend. Carbohyd. Polym. 2016, 136, 163–170. [Google Scholar] [CrossRef]
- Guo, J.; Liu, X.; Liu, M.; Han, M.; Liu, Y.; Ji, S. Effect of molecular weight of poly(ethylene glycol) on plasticization of poly(ʟ-lactic acid). Polymer 2021, 223, 123720. [Google Scholar] [CrossRef]
- Kim, C.-H.; Kim, D.-W.; Cho, K.Y. The influence of PEG molecular weight on the structural changes of corn starch in a starch/PEG blend. Polym. Bull. 2009, 63, 91–99. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Gollveia, R.F.; Carvalho, G.M.D.; Rubira, A.F.; Muniz, E.C. Polymer blends based on PEO and starch: Miscibility and spherulite growth rate evaluated through DSC and optical microscopy. Mater. Sci. Eng. C 2009, 29, 499–504. [Google Scholar] [CrossRef]
- Ning, W.; Jiugao, Y.; Xiaofei, M.; Chunmei, H. High performance modified thermoplastic starch/linear low-density polyethylene blends in one-step extrusion. Polym. Compos. 2007, 28, 89–97. [Google Scholar] [CrossRef]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Wang, B.; Yang, D.; Zhang, H.-R.; Huang, C.; Xiong, L.; Luo, J.; Chen, X.-D. Preparation of esterified bacterial cellulose for improved mechanical properties and the microstructure of isotactic polypropylene/bacterial cellulose composites. Polymers 2016, 8, 129. [Google Scholar] [CrossRef] [PubMed]









| CA Content (%wt) | Tg (°C) | Tcc (°C) | Tm (°C) | Xc (%) |
|---|---|---|---|---|
| - 1.5 3 4.5 | 42 42 42 42 | 77 77 79 80 | 159 157 158 157 | 19.5 19.7 16.5 16.1 |
| CA Content (%wt) | 50%-Td (°C) a | Residue Weight at 1000 °C (%) a | PLLA-Td,max (°C) b | PEG-Td,max (°C) b |
|---|---|---|---|---|
| - 1.5 3 4.5 | 353 356 357 364 | 3.98 3.86 4.22 3.94 | 359 359 361 367 | 416 414 415 416 |
| CA Content (%wt) | Water Contact Angle (°) | Ultimate Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|
| - 1.5 3 4.5 | 55.3 ± 4.5 51.1 ± 6.3 41.9 ± 4.2 35.5 ± 5.1 | 6.1 ± 0.7 7.4 ± 0.5 7.5 ± 1.2 8.3 ± 1.1 | 24 ± 6 51 ± 5 73 ± 7 80 ± 12 | 84 ± 15 89 ± 12 103 ± 14 107 ± 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srihanam, P.; Srisuwan, Y.; Phromsopha, T.; Manphae, A.; Baimark, Y. Improvement in Phase Compatibility and Mechanical Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/thermoplastic Starch Blends with Citric Acid. Polymers 2023, 15, 3966. https://doi.org/10.3390/polym15193966
Srihanam P, Srisuwan Y, Phromsopha T, Manphae A, Baimark Y. Improvement in Phase Compatibility and Mechanical Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/thermoplastic Starch Blends with Citric Acid. Polymers. 2023; 15(19):3966. https://doi.org/10.3390/polym15193966
Chicago/Turabian StyleSrihanam, Prasong, Yaowalak Srisuwan, Theeraphol Phromsopha, Apirada Manphae, and Yodthong Baimark. 2023. "Improvement in Phase Compatibility and Mechanical Properties of Poly(L-lactide)-b-poly(ethylene glycol)-b-poly(L-lactide)/thermoplastic Starch Blends with Citric Acid" Polymers 15, no. 19: 3966. https://doi.org/10.3390/polym15193966