Breaking the Barrier: Strategies for Mitigating Shuttle Effect in Lithium–Sulfur Batteries Using Advanced Separators
Abstract
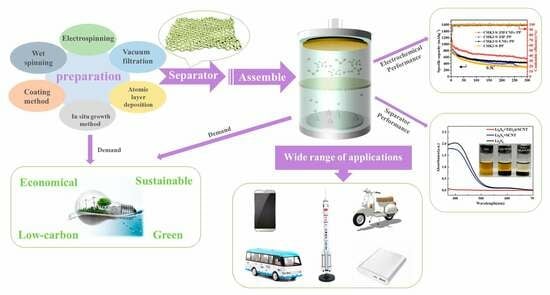
:1. Introduction

2. The Working Principle and Problems of Li-S
2.1. Working Mechanism of Li-S
2.2. The Existing Problems of Li-S
2.2.1. Cathode
2.2.2. Separator
2.2.3. Anode
2.2.4. Electrolyte
2.2.5. Binders
2.2.6. Current Collector

3. Methods
3.1. Electrospinning
3.2. Vacuum Filtration
3.3. Wet Spinning
3.4. Coating Method
3.5. In Situ Growth Method
3.6. Atomic Layer Deposition (ALD)
4. Application of Separators in Lithium–Sulfur Batteries
4.1. Electrospinning
4.1.1. Separator
4.1.2. Interlayer
4.2. Vacuum Filtration
4.2.1. Separator
4.2.2. Interlayer
4.3. Wet Spinning
4.4. Coating Method
4.4.1. Separator
4.4.2. Interlayer
4.5. In Situ Growth Method
4.5.1. Separator
4.5.2. Interlayer
4.6. Atomic Layer Deposition
4.6.1. Separator
4.6.2. Interlayer
4.7. Composite Process
4.7.1. Separator
4.7.2. Interlayer
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, N.; Lin, Y.X.; Yang, L.; Lu, D.P.; Xiao, J.; Qi, Y.; Cai, M. Cathode porosity is a missing key parameter to optimize lithium-sulfur battery energy density. Nat. Commun. 2019, 10, 4597. [Google Scholar] [CrossRef]
- Liu, M.; Deng, N.P.; Ju, J.G.; Fan, L.L.; Wang, L.Y.; Li, Z.J.; Zhao, H.J.; Yang, G.; Kang, W.M.; Yan, J.; et al. A Review: Electrospun Nanofiber Materials for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1905467. [Google Scholar] [CrossRef]
- Din, M.M.U.; Murugan, R. Metal Coated Polypropylene Separator with Enhanced Surface Wettability for High Capacity Lithium Metal Batteries. Sci. Rep. 2019, 9, 16795. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.C.; Kim, J.H.; Nam, S.; Park, C.R.; Yang, S.J. Rational Design of Nanostructured Functional Interlayer/Separator for Advanced Li-S Batteries. Adv. Funct. Mater. 2018, 28, 1707411. [Google Scholar] [CrossRef]
- Cuisinier, M.; Hart, C.; Balasubramanian, M.; Garsuch, A.; Nazar, L.F. Radical or Not Radical: Revisiting Lithium-Sulfur Electrochemistry in Nonaqueous Electrolytes. Adv. Energy Mater. 2015, 5, 1401801. [Google Scholar] [CrossRef]
- Yuan, Z.; Peng, H.J.; Hou, T.Z.; Huang, J.Q.; Chen, C.M.; Wang, D.W.; Cheng, X.B.; Wei, F.; Zhang, Q. Powering Lithium-Sulfur Battery Performance by Propelling Polysulfide Redox at Sulfiphilic Hosts. Nano Lett. 2016, 16, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Z.; Cai, W.L.; Kong, L.; Cai, J.S.; Zhang, Q.; Sun, J.Y. Rationalizing Electrocatalysis of Li-S Chemistry by Mediator Design: Progress and Prospects. Adv. Energy Mater. 2020, 10, 1901075. [Google Scholar] [CrossRef]
- Ren, W.C.; Ma, W.; Zhang, S.F.; Tang, B.T. Recent advances in shuttle effect inhibition for lithium sulfur batteries. Energy Storage Mater. 2019, 23, 707–732. [Google Scholar] [CrossRef]
- Liu, H.; Lai, W.-H.; Yang, H.-L.; Zhu, Y.-F.; Lei, Y.-J.; Zhao, L.; Peng, J.; Wang, Y.-X.; Chou, S.-L.; Liu, H.-K. Efficient separators with fast Li-ion transfer and high polysulfide entrapment for superior lithium-sulfur batteries. Chem. Eng. J. 2021, 408, 127348. [Google Scholar] [CrossRef]
- Balach, J.; Jaumann, T.; Klose, M.; Oswald, S.; Eckert, J.; Giebeler, L. Functional Mesoporous Carbon-Coated Separator for Long-Life, High-Energy Lithium-Sulfur Batteries. Adv. Funct. Mater. 2015, 25, 5285–5291. [Google Scholar] [CrossRef]
- Manthiram, A.; Chung, S.H.; Zu, C.X. Lithium-Sulfur Batteries: Progress and Prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lee, J.T.; Magasinski, A.; Kim, H.; Yushin, G. Solution-Based Processing of Graphene–Li2S Composite Cathodes for Lithium-Ion and Lithium–Sulfur Batteries. Part. Part. Syst. Charact. 2014, 31, 639–644. [Google Scholar] [CrossRef]
- Gu, S.; Huang, X.; Wang, Q.; Jin, J.; Wang, Q.; Wen, Z.; Qian, R. A hybrid electrolyte for long-life semi-solid-state lithium sulfur batteries. J. Mater. Chem. A 2017, 5, 13971–13975. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, L.; Wang, J.; Zhu, J.; Shen, P.K. In situ carbon nanotube clusters grown from three-dimensional porous graphene networks as efficient sulfur hosts for high-rate ultra-stable Li–S batteries. Nano Res. 2018, 11, 1731–1743. [Google Scholar] [CrossRef]
- Lee, W.Y.; Jin, E.M.; Cho, J.S.; Kang, D.-W.; Jin, B.; Jeong, S.M. Freestanding flexible multilayered Sulfur–Carbon nanotubes for Lithium–Sulfur battery cathodes. Energy 2020, 212, 118779. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.; Wu, F. From a historic review to horizons beyond: Lithium–sulphur batteries run on the wheels. Chem. Commun. 2015, 51, 18–33. [Google Scholar] [CrossRef]
- Phung, J.; Zhang, X.Z.; Deng, W.J.; Li, G. An overview of MOF-based separators for lithium-sulfur batteries. Sustain. Mater. Technol. 2022, 31, e00374. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Zhang, X.L.; Silva, S.R.P.; Ding, B.; Zhang, P.; Shao, G.S. Lithium-Sulfur Batteries Meet Electrospinning: Recent Advances and the Key Parameters for High Gravimetric and Volume Energy Density. Adv. Sci. 2022, 9, 2103879. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Yang, S.; Zhou, S.Y.; Zhang, L.B.; Gu, B.B.; Dong, Y.Y.; Kong, S.Z.; Cai, D.; Fang, G.Y.; Nie, H.G.; et al. Oxygen doping in antimony sulfide nanosheets to facilitate catalytic conversion of polysulfides for lithium-sulfur batteries. Chem. Commun. 2021, 57, 3255–3258. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Cuisinier, M.; Cabelguen, P.E.; Evers, S.; He, G.; Kolbeck, M.; Garsuch, A.; Bolin, T.; Balasubramanian, M.; Nazar, L.F. Sulfur Speciation in Li-S Batteries Determined by Operando X-ray Absorption Spectroscopy. J. Phys. Chem. Lett. 2013, 4, 3227–3232. [Google Scholar] [CrossRef]
- Diao, Y.; Xie, K.; Xiong, S.; Hong, X. Analysis of Polysulfide Dissolved in Electrolyte in Discharge-Charge Process of Li-S Battery. J. Electrochem. Soc. 2012, 159, A421–A425. [Google Scholar] [CrossRef]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Zhao, E.Y.; Nie, K.H.; Yu, X.Q.; Hu, Y.S.; Wang, F.W.; Xiao, J.; Li, H.; Huang, X.J. Advanced Characterization Techniques in Promoting Mechanism Understanding for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1707543. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Y.M.; Pasta, M.; Zhou, G.M.; Li, Y.Z.; Liu, W.; Xiong, F.; Cui, Y. Entrapment of Polysulfides by a Black-Phosphorus-Modified Separator for Lithium-Sulfur Batteries. Adv. Mater. 2016, 28, 9797–9803. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.S.; Cui, J.; Huang, J.Q.; Lu, Z.H.; Deng, Y.; Chong, W.G.; Wu, J.X.; Ihsan Ul Haq, M.; Ciucci, F.; Kim, J.K. Novel 2D Sb2S3 Nanosheet/CNT Coupling Layer for Exceptional Polysulfide Recycling Performance. Adv. Energy Mater. 2018, 8, 1800710. [Google Scholar] [CrossRef]
- Park, J.; Kim, E.T.; Kim, C.; Pyun, J.; Jang, H.S.; Shin, J.; Choi, J.W.; Char, K.; Sung, Y.E. The Importance of Confined Sulfur Nanodomains and Adjoining Electron Conductive Pathways in Subreaction Regimes of Li-S Batteries. Adv. Energy Mater. 2017, 7, 1700074. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zeng, H.X.; Li, X.W.; Pei, H.J.; Xue, Z.G.; Ye, Y.S.; Xie, X.L. Dual-Functional Interlayer Based on Radially Oriented Ultrathin MoS2 Nanosheets for High-Performance Lithium Sulfur-Batteries. Acs Appl. Energy Mater. 2019, 2, 1702–1711. [Google Scholar] [CrossRef]
- Zhao, M.; Li, B.-Q.; Chen, X.; Xie, J.; Yuan, H.; Huang, J.-Q. Redox Comediation with Organopolysulfides in Working Lithium-Sulfur Batteries. Chem 2020, 6, 3297–3311. [Google Scholar] [CrossRef]
- Zhao, M.; Peng, Y.-Q.; Li, B.-Q.; Zhang, X.-Q.; Huang, J.-Q. Regulation of carbon distribution to construct high-sulfur-content cathode in lithium–sulfur batteries. J. Energy Chem. 2021, 56, 203–208. [Google Scholar] [CrossRef]
- Sadd, M.; De Angelis, S.; Colding-Jorgensen, S.; Blanchard, D.; Johnsen, R.E.; Sanna, S.; Borisova, E.; Matic, A.; Bowen, J.R. Visualization of Dissolution-Precipitation Processes in Lithium-Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103126. [Google Scholar] [CrossRef]
- Ren, Y.X.; Zhao, T.S.; Liu, M.; Tan, P.; Zeng, Y.K. Modeling of lithium-sulfur batteries incorporating the effect of Li2S precipitation. J. Power Sources 2016, 336, 115–125. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous Hollow Carbon@Sulfur Composites for High-Power Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, Z.; Yu, Y.; Abruna, H.D.; Archer, L.A. Lithium-sulfur battery cathode enabled by lithium-nitrile interaction. J. Am. Chem. Soc. 2013, 135, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 2011, 11, 2644–2647. [Google Scholar] [CrossRef] [PubMed]
- Barai, P.; Mistry, A.; Mukherjee, P.P. Poromechanical effect in the lithium–sulfur battery cathode. Extrem. Mech. Lett. 2016, 9, 359–370. [Google Scholar] [CrossRef]
- Yan, J.H.; Liu, X.B.; Li, B.Y. Capacity Fade Analysis of Sulfur Cathodes in Lithium-Sulfur Batteries. Adv. Sci. 2016, 3, 1600101. [Google Scholar] [CrossRef]
- He, Y.B.; Qiao, Y.; Chang, Z.; Cao, X.; Jia, M.; He, P.; Zhou, H.S. Developing A “Polysulfide-Phobic” Strategy to Restrain Shuttle Effect in Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2019, 58, 11774–11778. [Google Scholar] [CrossRef]
- Liu, D.H.; Zhang, C.; Zhou, G.M.; Lv, W.; Ling, G.W.; Zhi, L.J.; Yang, Q.H. Catalytic Effects in Lithium-Sulfur Batteries: Promoted Sulfur Transformation and Reduced Shuttle Effect. Adv. Sci. 2018, 5, 1700270. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yi, S.; Liu, J.; Sun, S.; Liu, Y.; Yang, D.; Xi, K.; Gao, G.; Abdelkader, A.; Yan, W.; et al. Suppressing the Shuttle Effect and Dendrite Growth in Lithium-Sulfur Batteries. ACS Nano 2020, 14, 9819–9831. [Google Scholar] [CrossRef]
- Ghazi, Z.A.; He, X.; Khattak, A.M.; Khan, N.A.; Liang, B.; Iqbal, A.; Wang, J.X.; Sin, H.S.; Li, L.S.; Tang, Z.Y. MoS2/Celgard Separator as Efficient Polysulfide Barrier for Long-Life Lithium-Sulfur Batteries. Adv. Mater. 2017, 29, 1606817. [Google Scholar] [CrossRef]
- Zhai, P.; Liu, K.; Wang, Z.; Shi, L.; Yuan, S. Multifunctional separators for high-performance lithium-ion batteries. J. Power Sources 2021, 499, 229973. [Google Scholar] [CrossRef]
- Lei, T.; Chen, W.; Lv, W.; Huang, J.; Zhu, J.; Chu, J.; Yan, C.; Wu, C.; Yan, Y.; He, W.; et al. Inhibiting Polysulfide Shuttling with a Graphene Composite Separator for Highly Robust Lithium-Sulfur Batteries. Joule 2018, 2, 2091–2104. [Google Scholar] [CrossRef]
- Gao, Z.; Xue, Z.; Miao, Y.; Chen, B.; Xu, J.; Shi, H.; Tang, T.; Zhao, X. TiO2@Porous carbon nanotubes modified separator as polysulfide barrier for lithium-sulfur batteries. J. Alloys Compd. 2022, 906, 164249. [Google Scholar] [CrossRef]
- Cao, R.; Xu, W.; Lv, D.; Xiao, J.; Zhang, J.-G. Anodes for Rechargeable Lithium-Sulfur Batteries. Adv. Energy Mater. 2015, 5, 1402273. [Google Scholar] [CrossRef]
- Cheng, X.B.; Huang, J.Q.; Zhang, Q. Review-Li Metal Anode in Working Lithium-Sulfur Batteries. J. Electrochem. Soc. 2018, 165, A6058–A6072. [Google Scholar] [CrossRef]
- Xiong, S.; Xie, K.; Diao, Y.; Hong, X. Characterization of the solid electrolyte interphase on lithium anode for preventing the shuttle mechanism in lithium–sulfur batteries. J. Power Sources 2014, 246, 840–845. [Google Scholar] [CrossRef]
- Rong, G.L.; Zhang, X.Y.; Zhao, W.; Qiu, Y.C.; Liu, M.N.; Ye, F.M.; Xu, Y.; Chen, J.F.; Hou, Y.; Li, W.F.; et al. Liquid-Phase Electrochemical Scanning Electron Microscopy for In Situ Investigation of Lithium Dendrite Growth and Dissolution. Adv. Mater. 2017, 29, 1606187. [Google Scholar] [CrossRef]
- Rosso, M.; Brissot, C.; Teyssot, A.; Dolle, M.; Sannier, L.; Tarascon, J.M.; Bouchetc, R.; Lascaud, S. Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim. Acta 2006, 51, 5334–5340. [Google Scholar] [CrossRef]
- Fan, L.; Chen, S.H.; Zhu, J.Y.; Ma, R.F.; Li, S.P.; Podila, R.; Rao, A.M.; Yang, G.Z.; Wang, C.X.; Liu, Q.; et al. Simultaneous Suppression of the Dendrite Formation and Shuttle Effect in a Lithium-Sulfur Battery by Bilateral Solid Electrolyte Interface. Adv. Sci. 2018, 5, 1700934. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Lei, T.; Jiao, Y.; Wang, H.; Wang, X.; Rao, G.; Wang, X.; Chen, B.; Xiong, J. Graphene quantum dots as the nucleation sites and interfacial regulator to suppress lithium dendrites for high-loading lithium-sulfur battery. Nano Energy 2020, 68, 104373. [Google Scholar] [CrossRef]
- Akhtar, N.; Sun, X.; Yasir Akram, M.; Zaman, F.; Wang, W.; Wang, A.; Chen, L.; Zhang, H.; Guan, Y.; Huang, Y. A gelatin-based artificial SEI for lithium deposition regulation and polysulfide shuttle suppression in lithium-sulfur batteries. J. Energy Chem. 2021, 52, 310–317. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, S.; He, G.; Zhang, F. Novel Synergistic Strategy for Developing High-Performance Lithium Sulfur Batteries of Large Areal Sulfur Loading by SEI Modified Separator. ACS Appl. Energy Mater. 2018, 1, 932–940. [Google Scholar] [CrossRef]
- Li, G.; Huang, Q.; He, X.; Gao, Y.; Wang, D.; Kim, S.H.; Wang, D. Self-Formed Hybrid Interphase Layer on Lithium Metal for High-Performance Lithium-Sulfur Batteries. ACS Nano 2018, 12, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wen, Z.; Wu, M.; Jin, J.; Liu, Y. Vinylene carbonate–LiNO3: A hybrid additive in carbonic ester electrolytes for SEI modification on Li metal anode. Electrochem. Commun. 2015, 51, 59–63. [Google Scholar] [CrossRef]
- Li, N.W.; Yin, Y.X.; Yang, C.P.; Guo, Y.G. An Artificial Solid Electrolyte Interphase Layer for Stable Lithium Metal Anodes. Adv. Mater. 2016, 28, 1853–1858. [Google Scholar] [CrossRef]
- Bruckner, J.; Thieme, S.; Bottger-Hiller, F.; Bauer, I.; Grossmann, H.T.; Strubel, P.; Althues, H.; Spange, S.; Kaskel, S. Carbon- Based Anodes for Lithium Sulfur Full Cells with High Cycle Stability. Adv. Funct. Mater. 2014, 24, 1284–1289. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Yuan, S.; Wang, Y.; Xia, Y. To mitigate self-discharge of lithium–sulfur batteries by optimizing ionic liquid electrolytes. Energy Environ. Sci. 2016, 9, 224–231. [Google Scholar] [CrossRef]
- Jin, B.; Li, Y.; Qian, J.; Zhan, X.; Zhang, Q. Environmentally Friendly Binders for Lithium-Sulfur Batteries. ChemElectroChem 2020, 7, 4158–4176. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Liang, J.; Wu, K.; Xu, L.; Wang, J. Design of a high performance zeolite/polyimide composite separator for lithium-ion batteries. Polymers 2020, 12, 764. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Tan, Z. A review of electrospun nanofiber-based separators for rechargeable lithium-ion batteries. J. Power Sources 2019, 443, 227262. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, J.; Chong, C.B.; Yu, X.W.; Wanga, L.L.; Shi, Z.Q. An electrospun lignin/polyacrylonitrile nonwoven composite separator with high porosity and thermal stability for lithium-ion batteries. RSC Adv. 2015, 5, 101115–101120. [Google Scholar] [CrossRef]
- Rutledge, G.C.; Fridrikh, S.V. Formation of fibers by electrospinning. Adv. Drug Deliv. Rev. 2007, 59, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.L.; Kono, J. Science and applications of wafer-scale crystalline carbon nanotube films prepared through controlled vacuum filtration. R. Soc. Open Sci. 2019, 6, 181605. [Google Scholar] [CrossRef] [PubMed]
- Kritzer, P. Nonwoven support material for improved separators in Li–polymer batteries. J. Power Sources 2006, 161, 1335–1340. [Google Scholar] [CrossRef]
- Zhu, J.; Ge, Y.; Kim, D.; Lu, Y.; Chen, C.; Jiang, M.; Zhang, X. A novel separator coated by carbon for achieving exceptional high performance lithium-sulfur batteries. Nano Energy 2016, 20, 176–184. [Google Scholar] [CrossRef]
- Shao, H.; Wang, W.; Zhang, H.; Wang, A.; Chen, X.; Huang, Y. Nano-TiO2 decorated carbon coating on the separator to physically and chemically suppress the shuttle effect for lithium-sulfur battery. J. Power Sources 2018, 378, 537–545. [Google Scholar] [CrossRef]
- Yang, L.W.; Wang, Y.; Li, Q.; Li, Y.; Chen, Y.X.; Liu, Y.X.; Wu, Z.G.; Wang, G.K.; Zhong, B.H.; Song, Y.; et al. Inhibition of the shuttle effect of lithium-sulfur batteries via a tannic acid-metal one-step in situ chemical film-forming modified separator. Nanoscale 2021, 13, 5058–5068. [Google Scholar] [CrossRef]
- Lin, Q.Y.; Ding, B.; Chen, S.; Li, P.; Li, Z.W.; Shi, Y.Y.; Dou, H.; Zhang, X.G. Atomic Layer Deposition of Single Atomic Cobalt as a Catalytic Interlayer for Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 11206–11212. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Demir, M.M.; Yilgor, I.; Yilgor, E.; Erman, B. Electrospinning of polyurethane fibers. Polymer 2002, 43, 3303–3309. [Google Scholar] [CrossRef]
- Hao, J.; Lei, G.; Li, Z.; Wu, L.; Xiao, Q.; Wang, L. A novel polyethylene terephthalate nonwoven separator based on electrospinning technique for lithium ion battery. J. Membr. Sci. 2013, 428, 11–16. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Batchelor, W.; Varanasi, S.; Tsuzuki, T.; Wang, X.G. Effect of cellulose nanofiber dimensions on sheet forming through filtration. Cellulose 2012, 19, 561–574. [Google Scholar] [CrossRef]
- Cho, T.-H.; Tanaka, M.; Ohnishi, H.; Kondo, Y.; Yoshikazu, M.; Nakamura, T.; Sakai, T. Composite nonwoven separator for lithium-ion battery: Development and characterization. J. Power Sources 2010, 195, 4272–4277. [Google Scholar] [CrossRef]
- Safavi, A.; Fathi, S.; Babaei, M.R.; Mansoori, Z.; Latifi, M. Experimental and numerical analysis of fiber characteristics effects on fiber dispersion for wet-laid nonwoven. Fibers Polym. 2009, 10, 231–236. [Google Scholar] [CrossRef]
- Wang, X.R.; Yushin, G. Chemical vapor deposition and atomic layer deposition for advanced lithium ion batteries and supercapacitors. Energy Environ. Sci. 2015, 8, 1889–1904. [Google Scholar] [CrossRef]
- Yan, B.; Li, X.; Bai, Z.; Song, X.; Xiong, D.; Zhao, M.; Li, D.; Lu, S. A review of atomic layer deposition providing high performance lithium sulfur batteries. J. Power Sources 2017, 338, 34–48. [Google Scholar] [CrossRef]
- Zhu, X.B.; Ouyang, Y.; Chen, J.W.; Zhu, X.G.; Luo, X.; Lai, F.L.; Zhang, H.; Miao, Y.E.; Liu, T.X. In situ extracted poly(acrylic acid) contributing to electrospun nanofiber separators with precisely tuned pore structures for ultra-stable lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 3253–3263. [Google Scholar] [CrossRef]
- Guo, P.; Jiang, P.; Chen, W.; Qian, G.; He, D.; Lu, X. Bifunctional Al2O3/polyacrylonitrile membrane to suppress the growth of lithium dendrites and shuttling of polysulfides in lithium-sulfur batteries. Electrochim. Acta 2022, 428, 140955. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Pitcheri, R.; Zhu, J.; Wen, P.; Qiu, Y. Electrospun Ti4O7/C conductive nanofibers as interlayer for lithium-sulfur batteries with ultra long cycle life and high-rate capability. Chem. Eng. J. 2019, 355, 390–398. [Google Scholar] [CrossRef]
- Lin, Y.; Pitcheri, R.; Zhu, J.; Jiao, C.; Guo, Y.; Li, J.; Qiu, Y. Electrospun PVDF/PSSLi ionomer films as a functional separator for lithium-sulfur batteries. J. Alloys Compd. 2019, 785, 627–633. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, G.; Kang, W.; Deng, N.; Cheng, B. Taming polysulfides and facilitating lithium-ion migration: Novel electrospinning MOFs@PVDF-based composite separator with spiderweb-like structure for Li-S batteries. Electrochim. Acta 2021, 365, 137344. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhou, C.; Ling, M.; Lu, J.; Hou, Y.; Zhang, Q.; He, Q.; Zhan, X.; Chen, F. Flame-retardant and thermal-stable separator trapping polysulfides for lithium-sulfur battery. J. Alloys Compd. 2020, 826, 154197. [Google Scholar] [CrossRef]
- Deng, N.; Wang, Y.; Yan, J.; Ju, J.; Li, Z.; Fan, L.; Zhao, H.; Kang, W.; Cheng, B. A F-doped tree-like nanofiber structural poly-m-phenyleneisophthalamide separator for high-performance lithium-sulfur batteries. J. Power Sources 2017, 362, 243–249. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, X.; Li, M.; Ji, Y.; Li, Q.; He, X.; Lei, Z.; Liu, Z.; Jiang, R.; Sun, J. Synthesis of Titanium Molybdenum Nitride-Decorated Electrospun Carbon Nanofiber Membranes as Interlayers to Suppress Polysulfide Shuttling in Lithium–Sulfur Batteries. ACS Sustain. Chem. Eng. 2022, 10, 776–788. [Google Scholar] [CrossRef]
- Du, X.; Ma, D.; Zhang, Y.; Ma, J.; Wang, J.; Xiao, Q.; Wang, B.; Tian, L.; Zhuang, J. Electrospun TiO2 Nanofibers Featuring Surface Oxygen Vacancies as a Multifunctional Interlayer for High-Performance Lithium–Sulfur Batteries in a Wide Temperature Range. Inorg. Chem. 2023, 62, 5134–5144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, S.; Zou, B.; Li, G.; Yang, S.; Zhao, Y.; Lian, J.; Li, H.; Ji, H. Electrospun CoSe@NC nanofiber membrane as an effective polysulfides adsorption-catalysis interlayer for Li-S batteries. Chem. Eng. J. 2022, 430, 131911. [Google Scholar] [CrossRef]
- Huangfu, Y.; Zheng, T.; Zhang, K.; She, X.; Xu, H.; Fang, Z.; Xie, K. Facile fabrication of permselective g-C3N4 separator for improved lithium-sulfur batteries. Electrochim. Acta 2018, 272, 60–67. [Google Scholar] [CrossRef]
- Feng, P.; Hou, W.; Bai, Z.; Bai, Y.; Sun, K.; Wang, Z. Ultrathin two-dimensional bimetal NiCo-based MOF nanosheets as ultralight interlayer in lithium-sulfur batteries. Chin. Chem. Lett. 2023, 34, 107427. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Fan, Z.; Li, S.; Bernussi, A.; Newman, N. Functionalized bacterial cellulose as a separator to address polysulfides shuttling in lithium–sulfur batteries. Mater. Today Energy 2021, 21, 100813. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Lai, Y.; Li, J. A freestanding hollow carbon nanofiber/reduced graphene oxide interlayer for high-performance lithium–sulfur batteries. J. Alloys Compd. 2016, 663, 501–506. [Google Scholar] [CrossRef]
- Zheng, B.; Yu, L.; Zhao, Y.; Xi, J. Ultralight carbon flakes modified separator as an effective polysulfide barrier for lithium-sulfur batteries. Electrochim. Acta 2019, 295, 910–917. [Google Scholar] [CrossRef]
- Liu, Q.; Han, X.T.; Dou, Q.Y.; Xiong, P.X.; Kang, Y.B.; Kang, S.W.; Kim, B.K.; Park, H.S. Multiphase and Multicomponent Nickel-Iron Oxide Heterostructure as an Efficient Separator Modification Layer for Advanced Lithium Sulfur Batteries. Batter. Supercaps 2021, 4, 1843–1849. [Google Scholar] [CrossRef]
- Tian, M.; Pei, F.; Yao, M.; Fu, Z.; Lin, L.; Wu, G.; Xu, G.; Kitagawa, H.; Fang, X. Ultrathin MOF nanosheet assembled highly oriented microporous membrane as an interlayer for lithium-sulfur batteries. Energy Storage Mater. 2019, 21, 14–21. [Google Scholar] [CrossRef]
- Sung, S.; Kim, B.H.; Lee, S.; Choi, S.; Yoon, W.Y. Increasing sulfur utilization in lithium-sulfur batteries by a Co-MOF-74@MWCNT interlayer. J. Energy Chem. 2021, 60, 186–193. [Google Scholar] [CrossRef]
- Meng, L.; Li, Y.; Lin, Q.X.; Long, J.; Wang, Y.; Hu, J. Nitrogen and Oxygen Dual Self-Doped Flexible PPTA Nanofiber Carbon Paper as an Effective Interlayer for Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2021, 4, 8592–8603. [Google Scholar] [CrossRef]
- Li, Y.; Meng, L.; Jin, L.; Yun, L.; Jian, H. A wet-laid carbon paper with 3D conductive structure as an interlayer for lithium-sulfur batteries. Mater. Res. Express 2019, 6, 125547. [Google Scholar] [CrossRef]
- Chong, W.G.; Xiao, F.; Yao, S.S.; Cui, J.; Sadighi, Z.; Wu, J.X.; Ihsan-Ul-Haq, M.; Shao, M.H.; Kim, J.K. Nitrogen-doped graphene fiber webs for multi-battery energy storage. Nanoscale 2019, 11, 6334–6342. [Google Scholar] [CrossRef]
- Suriyakumar, S.; Stephan, A.M. Mitigation of Polysulfide Shuttling by Interlayer/Permselective Separators in Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 8095–8129. [Google Scholar] [CrossRef]
- Chong, W.G.; Huang, J.Q.; Xu, Z.L.; Qin, X.Y.; Wang, X.Y.; Kim, J.K. Lithium-Sulfur Battery Cable Made from Ultralight, Flexible Graphene/Carbon Nanotube/Sulfur Composite Fibers. Adv. Funct. Mater. 2017, 27, 1604815. [Google Scholar] [CrossRef]
- Chong, W.G.; Xiao, Y.; Huang, J.-Q.; Yao, S.; Cui, J.; Qin, L.; Gao, C.; Kim, J.-K. Highly conductive porous graphene/sulfur composite ribbon electrodes for flexible lithium-sulfur batteries. Nanoscale 2018, 10, 21132–21141. [Google Scholar] [CrossRef]
- Zhang, S.M.; Shi, H.D.; Tang, J.W.; Shi, W.X.; Wu, Z.S.; Wang, X. Super-aligned films of sub-1 nm Bi2O3-polyoxometalate nanowires as interlayers in lithium-sulfur batteries. Sci. China-Mater. 2021, 64, 2949–2957. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Zhang, S.; Gu, S.; Jin, J.; Wen, Z. Metal-organic-framework-derived N-C-Co film as a shuttle-suppressing interlayer for lithium sulfur battery. Chem. Eng. J. 2018, 334, 2356–2362. [Google Scholar] [CrossRef]
- Zhang, Z.; Lai, Y.; Zhang, Z.; Zhang, K.; Li, J. Al2O3-coated porous separator for enhanced electrochemical performance of lithium sulfur batteries. Electrochim. Acta 2014, 129, 55–61. [Google Scholar] [CrossRef]
- Hu, X.; Huang, T.; Wang, S.; Lin, S.; Feng, Z.; Chung, L.-H.; He, J. Separator modified by Co-porphyrin based Zr-MOF@CNT composite enabling efficient polysulfides catalytic conversion for advanced lithium-sulfur batteries. Electrochim. Acta 2021, 398, 139317. [Google Scholar] [CrossRef]
- Ma, B.; Gao, Y.; Niu, M.; Luo, M.; Li, H.; Bai, Y.; Sun, K. ZIF-67/Super P modified separator as an efficient polysulfide barrier for high-performance lithium-sulfur batteries. Solid State Ion. 2021, 371, 115750. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, M.; Sun, H.; Li, G.; Fu, Q.; Li, H.; Liu, J.; Sun, L.; Mauger, A.; Julien, C.M.; et al. Constructing metal-free and cost-effective multifunctional separator for high-performance lithium-sulfur batteries. Nano Energy 2019, 59, 390–398. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Q.; An, Y.; Chen, R.; Sun, N.; Wu, F.; Xu, B. A 3D conductive carbon interlayer with ultrahigh adsorption capability for lithium-sulfur batteries. Appl. Surf. Sci. 2018, 440, 770–777. [Google Scholar] [CrossRef]
- Li, W.; Ye, Y.; Qian, J.; Xing, Y.; Qu, W.; Zhang, N.; Li, L.; Wu, F.; Chen, R. Oxygenated Nitrogen-Doped Microporous Nanocarbon as a Permselective Interlayer for Ultrastable Lithium-Sulfur Batteries. ChemElectroChem 2019, 6, 1094–1100. [Google Scholar] [CrossRef]
- Lu, X.; Wang, H.; Liu, X.; Song, Z.; Jiang, N.; Xie, F.; Zheng, Q.; Lin, D. Functional separators prepared via in-situ growth of hollow CoSO4 hydrate arrays on pristine polypropylene membrane for high performance lithium-Sulfur batteries. J. Alloys Compd. 2020, 838, 155618. [Google Scholar] [CrossRef]
- Li, J.; Jiao, C.; Zhu, J.; Zhong, L.; Kang, T.; Aslam, S.; Wang, J.; Zhao, S.; Qiu, Y. Hybrid co-based MOF nanoboxes/CNFs interlayer as microreactors for polysulfides-trapping in lithium-sulfur batteries. J. Energy Chem. 2021, 57, 469–476. [Google Scholar] [CrossRef]
- Liu, J.W.; Wang, J.A.; Zhu, L.; Chen, X.; Yi, G.; Ma, Q.Y.; Sun, S.Y.; Wang, N.; Cui, X.M.; Chai, Q.Q.; et al. In situ grown MOFs and PVDF-HFP co-modified aramid gel nanofiber separator for high-safety lithium-sulfur batteries. J. Mater. Chem. A 2022, 10, 14098–14110. [Google Scholar] [CrossRef]
- Zhang, F.; Gao, Y.; Wu, F.; Li, L.; Li, J.; Wang, G. Constructing MIL-101(Cr) membranes on carbon nanotube films as ion-selective interlayers for lithium–sulfur batteries. Nanotechnology 2022, 33, 215401. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Wu, F.; Jin, G.; Li, J.; Zhang, Z. Continuous zirconium-based MOF-808 membranes for polysulfide shuttle suppression in lithium-sulfur batteries. Appl. Surf. Sci. 2022, 596, 153628. [Google Scholar] [CrossRef]
- Yi, R.; Liu, C.; Zhao, Y.; Hardwick, L.J.; Li, Y.; Geng, X.; Zhang, Q.; Yang, L.; Zhao, C. A light-weight free-standing graphene foam-based interlayer towards improved Li-S cells. Electrochim. Acta 2019, 299, 479–488. [Google Scholar] [CrossRef]
- Han, X.; Xu, Y.; Chen, X.; Chen, Y.-C.; Weadock, N.; Wan, J.; Zhu, H.; Liu, Y.; Li, H.; Rubloff, G.; et al. Reactivation of dissolved polysulfides in Li–S batteries based on atomic layer deposition of Al2O3 in nanoporous carbon cloth. Nano Energy 2013, 2, 1197–1206. [Google Scholar] [CrossRef]
- Kong, W.; Wang, D.; Yan, L.; Luo, Y.; Jiang, K.; Li, Q.; Zhang, L.; Lu, S.; Fan, S.; Li, J.; et al. Ultrathin HfO2-modified carbon nanotube films as efficient polysulfide barriers for Li-S batteries. Carbon 2018, 139, 896–905. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Q.; Liu, Z.; Wang, J.; Ma, R.; Fan, L.; Wang, T.; Zhao, J.; Ge, J.; Lu, X.; et al. TiO2 quantum dots decorated multi-walled carbon nanotubes as the multifunctional separator for highly stable lithium sulfur batteries. Electrochim. Acta 2018, 284, 314–320. [Google Scholar] [CrossRef]
- Moon, S.-H.; Kim, M.-C.; Choi, J.-H.; Kim, Y.-S.; Kim, H.; Park, K.-W. 1T-MoS2/carbon nanofiber composite as an interlayer fabricated by an in situ electrochemical fabrication method for lithium-sulfur batteries. J. Alloys Compd. 2021, 857, 158236. [Google Scholar] [CrossRef]
- Leng, X.; Zeng, J.; Yang, M.; Li, C.; Vattikuti, S.V.P.; Chen, J.; Li, S.; Shim, J.; Guo, T.; Ko, T.J. Bimetallic Ni–Co MOF@PAN modified electrospun separator enhances high-performance lithium-sulfur batteries. J. Energy Chem. 2023, 82, 484–496. [Google Scholar] [CrossRef]
- Kiai, M.S.; Eroglu, O.; Kizil, H. Electrospun nanofiber polyacrylonitrile coated separators to suppress the shuttle effect for long-life lithium–sulfur battery. J. Appl. Polym. Sci. 2020, 137, 48606. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Fu, Y. A thermoregulating separator based on black phosphorus/MOFs heterostructure for thermo-stable lithium-sulfur batteries. Chem. Eng. J. 2023, 454, 140250. [Google Scholar] [CrossRef]
- Wang, H.; Xu, C.; Du, X.; Liu, G.; Han, W.; Li, J. Ordered porous metal oxide embedded dense carbon network design as high-performance interlayer for stable lithium-sulfur batteries. Chem. Eng. J. 2023, 471, 144338. [Google Scholar] [CrossRef]
- Wang, L.; He, Y.-B.; Shen, L.; Lei, D.; Ma, J.; Ye, H.; Shi, K.; Li, B.; Kang, F. Ultra-small self-discharge and stable lithium-sulfur batteries achieved by synergetic effects of multicomponent sandwich-type composite interlayer. Nano Energy 2018, 50, 367–375. [Google Scholar] [CrossRef]














| Main Materials | Initial Capacity (mAh g−1) | Capacity Remaining (mAh g−1) | Decay Rate | SEM Figure | Reference | |
|---|---|---|---|---|---|---|
| Separator | PVDF/PSSLi | 955 | 466 (0.5 C, 200 cycles) | 0.26% |  | [82] |
| PVDF/MOF | 1324.2 | 551 (2 C, 700 cycles) | 0.05% |  | [83] | |
| PI/MC | 1602.3 | 905.5 (0.2 C, 100 cycles) | --- |  | [84] | |
| PMIA | 1222.25 | 745.7 (0.5 C, 800 cycles) | --- |  | [85] | |
| Interlayer | TMN@CNF | 947 | 390 (2 C, 1000 cycles) | 0.059% |  | [86] |
| TCNF | 1279 | 798 (2.5 A g−1, 1000 cycles) | 0.057% |  | [87] | |
| CoSe@NC | 1317 | 804.7 (0.1 C, 100 cycles) | --- |  | [88] |
| Main Materials | Initial Capacity (mAh g−1) | Capacity Remaining (mAh g−1) | Decay Rate | SEM Figure | Reference | |
|---|---|---|---|---|---|---|
| Separator | BC | 1175 | 735 (0.3 C, 300 cycles) | 0.07% |  | [91] |
| HCNF/rGO | 1318.4 | 533.6 (1 C, 400 cycles) | 0.13% |  | [92] | |
| CF/PP | 1111 | 683 (0.5 C, 500 cycles) | 0.071% |  | [93] | |
| NiFe2O4–NiO/PP | 1350 | 755 (2 C, 1000 cycles) | 0.065% |  | [94] | |
| Interlayer | MOF | 850 | 604 (1 C, 900 cycles) | 0.032% |  | [95] |
| Co-MOF-74@MWCNT | 1434 | 771 (0.1 C, 200 cycles) | --- |  | [96] |
| Main Materials | Initial Capacity (mAh g−1) | Capacity Remaining (mAh g−1) | Decay Rate | SEM Figure | Reference | |
|---|---|---|---|---|---|---|
| Separator | Al2O3/PP | 967 | 593.4 (0.5 C, 50 cycles) | 0.13% |  | [105] |
| Zr-MOF@CNT/PP | 1157 | 545 (1 C, 500 cycles) | 0.067% |  | [106] | |
| ZIF-67/PP | 1341 | 761 (0.2 C, 300 cycles) | 0.14% |  | [107] | |
| RP/PP | 1287 | 729.6 (1 C, 500 cycles) | 0.109% |  | [108] | |
| Interlayer | CFF | 1346.9 | 1076.6 (0.1 C, 100 cycles) | --- |  | [109] |
| OMNC | 994.4 | 587.6 (0.5 C, 100 cycles) | --- |  | [110] |
| Main Materials | Initial Capacity (mAh g−1) | Capacity Remaining (mAh g−1) | Decay Rate | SEM Figure | Reference | |
|---|---|---|---|---|---|---|
| Separator | TA-Co/PP | 1182 | 549.9 (2 C, 500 cycles) | 0.065% |  | [69] |
| Z-PMIA | 1391.2 | 961.1 (0.2 C, 350 cycles) | 0.033% |  | [55] | |
| PMIA/ZIF-8 | 1156 | 855 (0.2 C, 300 cycles) | 0.086% |  | [113] | |
| Interlayer | MIL-101/CNT | 816 | 628 (1 C, 500 cycles) | 0.046% |  | [114] |
| MOF-808 | 908.1 | 755.5 (1 C, 500 cycle) | 0.03% |  | [115] |
| Main Materials | Initial Capacity (mAh g−1) | Capacity Remaining (mAh g−1) | Decay Rate | SEM Figure | Reference | |
|---|---|---|---|---|---|---|
| Interlayer | ALD-ZnO | 998 | 846 (0.2 C,100 cycles) | --- |  | [116] |
| Al2O3 | 1136 | 766 (40 cycles) | --- |  | [117] | |
| HfO2-CNT | 1275 | 995 (0.2 C, 100 cycles) | --- |  | [118] |
| Main Materials | Initial Capacity (mAh g−1) | Capacity Remaining (mAh g−1) | Decay Rate | SEM Figure | Reference | |
|---|---|---|---|---|---|---|
| Separator | Ni-Co MOF@PAN | 944 | 794 (2 C, 2000 cycles) | 0.034% |  | [121] |
| PAN/PDAAQ | 881 | 766 (1 C, 800 cycles) | 0.11% |  | [122] | |
| UIO66@BP/PAN | --- | 761 (0.5 C, 1000 cycles) | 0.016% |  | [123] | |
| Interlayer | N, Co-TiO x/NCNT@CNFs | 1132 | 988 (0.2 C, 100 cycles) | --- |  | [124] |
| CNF@VS 2/CNT | 834 | 605 (1 C, 1145 cycles) | --- |  | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Chen, Z.; Chen, H.; Fu, X.; Awuye, D.E.; Yin, X.; Zhao, Y. Breaking the Barrier: Strategies for Mitigating Shuttle Effect in Lithium–Sulfur Batteries Using Advanced Separators. Polymers 2023, 15, 3955. https://doi.org/10.3390/polym15193955
Zhu Y, Chen Z, Chen H, Fu X, Awuye DE, Yin X, Zhao Y. Breaking the Barrier: Strategies for Mitigating Shuttle Effect in Lithium–Sulfur Batteries Using Advanced Separators. Polymers. 2023; 15(19):3955. https://doi.org/10.3390/polym15193955
Chicago/Turabian StyleZhu, Yingbao, Zhou Chen, Hui Chen, Xuguang Fu, Desire Emefa Awuye, Xichen Yin, and Yixuan Zhao. 2023. "Breaking the Barrier: Strategies for Mitigating Shuttle Effect in Lithium–Sulfur Batteries Using Advanced Separators" Polymers 15, no. 19: 3955. https://doi.org/10.3390/polym15193955