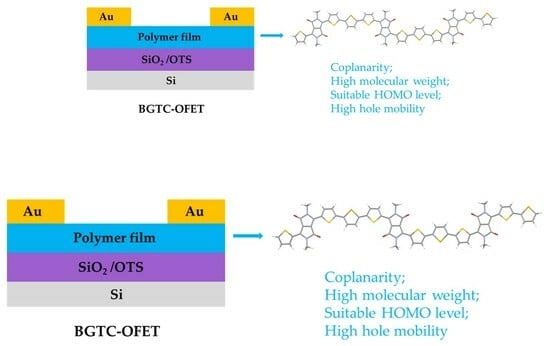
Donor-Acceptor-Based Organic Polymer Semiconductor Materials to Achieve High Hole Mobility in Organic Field-Effect Transistors
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Synthesis
3.2. Density Functional Theory Calculations
3.3. Photophysical and Electrochemical Properties
3.4. OFET Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, D.; Zhang, X.; Nwachukwu, C.I.; Miller, E.J.; Hansen, K.R.; Flannery, L.; Ogle, J.; Berzansky, A.; Labram, J.G.; Roberts, A.G.; et al. Establishing Self-Dopant Design Principles from Structure–Function Relationships in Self-n-Doped Perylene Diimide Organic Semiconductors. Adv. Mater. 2022, 34, 2204656–2204668. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, C. Organic optoelectronics creating new opportunities for science and applications. Front. Optoelectron. 2022, 15, 51–53. [Google Scholar] [CrossRef]
- Sirringhaus, H. 25th Anniversary Article: Organic Field-Effect Transistors: The Path Beyond Amorphous Silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, H.; Nielsen, C.B.; Schroeder, B.C.; McCulloch, I. The role of chemical design in the performance of organic semiconductors. Nat. Rev. Chem. 2020, 4, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Virkar, A.A.; Mannsfeld, S.; Bao, Z.; Stingelin, N. Organic Semiconductor Growth and Morphology Considerations for Organic Thin-Film Transistors. Adv. Mater. 2010, 22, 3857–3875. [Google Scholar] [CrossRef]
- Becerril, H.A.; Roberts, M.E.; Liu, Z.; Locklin, J.; Bao, Z. High-Performance Organic Thin-Film Transistors through Solution-Sheared Deposition of Small-Molecule Organic Semiconductors. Adv. Mater. 2008, 20, 2588–2594. [Google Scholar] [CrossRef]
- Sun, H.; Guo, X.; Facchetti, A. High-Performance n-Type Polymer Semiconductors: Applications, Recent Development, and Challenges. Chem 2020, 6, 1310–1326. [Google Scholar] [CrossRef]
- Fratini, S.; Nikolka, M.; Salleo, A.; Schweicher, G.; Sirringhaus, H. Charge transport in high-mobility conjugated polymers and molecular semiconductors. Nat. Mater. 2020, 19, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Jha, P.; Singh, A.; Chehimi, M.M.; Aswal, D.K. Flexible organic semiconductor thin films. J. Mater. Chem. C 2015, 3, 8468–8479. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Hu, W. Organic field-effect transistor-based gas sensors. Chem. Soc. Rev. 2015, 44, 2087–2107. [Google Scholar] [CrossRef]
- Shaposhnik, P.A.; Zapunidi, S.A.; Shestakov, M.V.; Agina, E.V.; Ponomarenko, S.A. Modern bio and chemical sensors and neuromorphic devices based on organic semiconductors. Russ. Chem. Rev. 2020, 89, 1483–1506. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Y.; Zhu, D. π-Conjugated molecules with fused rings for organic field-effect transistors: Design, synthesis and applications. Chem. Soc. Rev. 2010, 39, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Di, C.-a. Exploring Thermoelectric Materials from High Mobility Organic Semiconductors. Chem. Mater. 2020, 32, 2688–2702. [Google Scholar] [CrossRef]
- Jia, H.; Lei, T. Emerging research directions for n-type conjugated polymers. J. Mater. Chem. C 2019, 7, 12809–12821. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 17086–17100. [Google Scholar] [CrossRef]
- Li, Y.; Sonar, P.; Murphy, L.; Hong, W. High mobility diketopyrrolopyrrole (DPP)-based organic semiconductor materials for organic thin film transistors and photovoltaics. Energy Environ. Sci. 2013, 6, 1684–1711. [Google Scholar] [CrossRef]
- Ren, S.; Yassar, A. Recent Research Progress in Indophenine-Based-Functional Materials: Design, Synthesis, and Optoelectronic Applications. Materials 2023, 16, 2474. [Google Scholar] [CrossRef]
- Ren, S.; Habibi, A.; Ni, P.; Nahdi, H.; Bouanis, F.Z.; Bourcier, S.; Clavier, G.; Frigoli, M.; Yassar, A. Synthesis and characterization of solution-processed indophenine derivatives for function as a hole transport layer for perovskite solar cells. Dye. Pigment. 2023, 213, 111136. [Google Scholar] [CrossRef]
- Chen, Z.; Li, M.; Hu, M.; Wang, S.; Miao, Z.; Xu, S.; Chen, C.; Dong, H.; Huang, W.; Chen, R. All-acceptor polymers with noncovalent interactions for efficient ambipolar transistors. J. Mater. Chem. C 2020, 8, 2094–2101. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, W.; Wang, Z.; Yassar, A.; Liao, Z.; Yi, Z. Synergistic Use of All-Acceptor Strategies for the Preparation of an Organic Semiconductor and the Realization of High Electron Transport Properties in Organic Field-Effect Transistors. Polymers 2023, 15, 3392. [Google Scholar] [CrossRef]
- Yi, Z.; Yan, Y.; Wang, H.; Li, W.; Liu, K.; Zhao, Y.; Gu, G.; Liu, Y. Chain-Extending Polymerization for Significant Improvement in Organic Thin-Film Transistor Performance. ACS Appl. Mater. Interfaces 2022, 14, 36918–36926. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Guo, Y.; Liu, Y. Acceptor Modulation Strategies for Improving the Electron Transport in High-Performance Organic Field-Effect Transistors. Adv. Mater. 2021, 34, 2104325–2104355. [Google Scholar] [CrossRef] [PubMed]
- Stas, S.; Sergeyev, S.; Geerts, Y. Synthesis of diketopyrrolopyrrole (DPP) derivatives comprising bithiophene moieties. Tetrahedron 2010, 66, 1837–1845. [Google Scholar] [CrossRef]
- Luo, N.; Zhang, G.; Liu, Z. Keep glowing and going: Recent progress in diketopyrrolopyrrole synthesis towards organic optoelectronic materials. Org. Chem. Front. 2021, 8, 4560–4581. [Google Scholar] [CrossRef]
- Shi, L.; Guo, Y.; Hu, W.; Liu, Y. Design and effective synthesis methods for high-performance polymer semiconductors in organic field-effect transistors. Mater. Chem. Front. 2017, 1, 2423–2456. [Google Scholar] [CrossRef]
- Lee, M.Y.; Dharmapurikar, S.; Lee, S.J.; Cho, Y.; Yang, C.; Oh, J.H. Regular H-Bonding-Containing Polymers with Stretchability up to 100% External Strain for Self-Healable Plastic Transistors. Chem. Mater. 2020, 32, 1914–1924. [Google Scholar] [CrossRef]
- Tunega, D.; Bucko, T.; Zaoui, A. Assessment of ten DFT methods in predicting structures of sheet silicates: Importance of dispersion corrections. J. Chem. Phys. 2012, 137, 114105–114115. [Google Scholar] [CrossRef]
- Goerigk, L. How Do DFT-DCP, DFT-NL, and DFT-D3 Compare for the Description of London-Dispersion Effects in Conformers and General Thermochemistry? J. Chem. Theory Comput. 2014, 10, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Cao, Y.; Fan, Y.; Liu, C.-J.; Yuan, S.-C.; Pei, J. High-Performance Air-Stable Organic Field-Effect Transistors: Isoindigo-Based Conjugated Polymers. J. Am. Chem. Soc. 2011, 133, 6099–6101. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Y.; Yang, J.; Sun, Y.; Shi, L.; Ran, Y.; Zhang, Q.; Yi, Y.; Wang, S.; Guo, Y.; et al. Copolymers of Bis-Diketopyrrolopyrrole and Benzothiadiazole Derivatives for High-Performance Ambipolar Field-Effect Transistors on Flexible Substrates. ACS Appl. Mater. Interfaces 2018, 10, 25858–25865. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing. J. Chem. Phys. 1996, 104, 1040–1046. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Lin, Y.-C.; Chen, C.-H.; Wei, K.-H.; Su, Y.-W.; Chen, P.-T. Density functional theory study of donor–acceptor conjugated polymers with substituent effect. J. Polym. Res. 2021, 28, 427–437. [Google Scholar] [CrossRef]
- Ren, S.; Habibi, A.; Wang, Y.; Yassar, A. Investigating the Effect of Cross-Conjugation Patterns on the Optoelectronic Properties of 7,7′Isoindigo-Based Materials. Electronics 2023, 12, 3313. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, M.; Shao, M.; Shi, W.; Yang, J.; Kuang, J.; Wang, C.; Gao, W.; Zhu, C.; Meng, R.; et al. Molecular Design of Multifunctional Integrated Polymer Semiconductors with Intrinsic Stretchability, High Mobility, and Intense Luminescence. Adv. Mater. 2023, e2305987–e2306021. [Google Scholar] [CrossRef]
- Li, Y.; Sonar, P.; Singh, S.P.; Soh, M.S.; van Meurs, M.; Tan, J. Annealing-free high-mobility diketopyrrolopyrrole-quaterthiophene copolymer for solution-processed organic thin film transistors. J. Am. Chem. Soc. 2011, 133, 2198–2204. [Google Scholar] [CrossRef]
- Park, G.E.; Shin, J.; Lee, D.H.; Lee, T.W.; Shim, H.; Cho, M.J.; Pyo, S.; Choi, D.H. Acene-Containing Donor–Acceptor Conjugated Polymers: Correlation between the Structure of Donor Moiety, Charge Carrier Mobility, and Charge Transport Dynamics in Electronic Devices. Macromolecules 2014, 47, 3747–3754. [Google Scholar] [CrossRef]
- Park, J.H.; Jung, E.H.; Jung, J.W.; Jo, W.H. A fluorinated phenylene unit as a building block for high-performance n-type semiconducting polymer. Adv. Mater. 2013, 25, 2583–2588. [Google Scholar] [CrossRef]
- Yuan, Z.; Fu, B.; Thomas, S.; Zhang, S.; DeLuca, G.; Chang, R.; Lopez, L.; Fares, C.; Zhang, G.; Bredas, J.-L.; et al. Unipolar Electron Transport Polymers: A Thiazole Based All-Electron Acceptor Approach. Chem. Mater. 2016, 28, 6045–6049. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Kohara, A.; Matsumoto, H.; Manzhos, S.; Feron, K.; Bottle, S.E.; Bell, J.; Michinobu, T.; Sonar, P. Tuning the Charge Carrier Polarity of Organic Transistors by Varying the Electron Affinity of the Flanked Units in Diketopyrrolopyrrole-Based Copolymers. Adv. Funct. Mater. 2019, 30, 1907452–1907461. [Google Scholar] [CrossRef]










| C | H | N | S | |
|---|---|---|---|---|
| (%) | (%) | (%) | (%) | |
| theoretical value 1 | 71.89 | 9.69 | 2.54 | 5.81 |
| 1st experimental value | 71.42 | 9.60 | 2.28 | 6.20 |
| 2nd experimental value | 71.67 | 9.68 | 2.31 | 6.21 |
| average value | 71.54 | 9.64 | 2.30 | 6.20 |
| ε (cm−1 M−1) | fosc | λmax caclu (nm) | λonset caclu (nm) | Eopt 2 (eV) | HOMO (eV) | LUMO (eV) | Ec 3 (eV) | |
|---|---|---|---|---|---|---|---|---|
| PDPP-2S-Se 1 | 265605 | 2.97 | 825 | 995 | 1.25 | −4.94 | −3.19 | 1.75 |
| λmaxsoln (nm) | λmaxfilm (nm) | Egsoln (eV) 1 | Egfilm (eV) 1 | LUMO (eV) 2 | HOMO (eV) 3 | Egcv (eV) 4 | |
|---|---|---|---|---|---|---|---|
| PDPP-2S-Se | 765 | 884 | 1.45 | 1.22 | −3.79 | −5.29 | 1.50 |
| Rotation Speed (mm/s) | Annealing Temperature (°C) | Hole Mobilities 1 (cm2/(V s)) | Max Mobilities (cm2/(V s)) | Threshold Voltages (V) | ION/OFF | |
|---|---|---|---|---|---|---|
| PDPPTT-2Tz | 2000 | 220 | 0.39 | 0.59 | 0.2 | 104–105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Wang, Z.; Zhang, W.; Ding, Y.; Yi, Z. Donor-Acceptor-Based Organic Polymer Semiconductor Materials to Achieve High Hole Mobility in Organic Field-Effect Transistors. Polymers 2023, 15, 3713. https://doi.org/10.3390/polym15183713
Ren S, Wang Z, Zhang W, Ding Y, Yi Z. Donor-Acceptor-Based Organic Polymer Semiconductor Materials to Achieve High Hole Mobility in Organic Field-Effect Transistors. Polymers. 2023; 15(18):3713. https://doi.org/10.3390/polym15183713
Chicago/Turabian StyleRen, Shiwei, Zhuoer Wang, Wenqing Zhang, Yubing Ding, and Zhengran Yi. 2023. "Donor-Acceptor-Based Organic Polymer Semiconductor Materials to Achieve High Hole Mobility in Organic Field-Effect Transistors" Polymers 15, no. 18: 3713. https://doi.org/10.3390/polym15183713