Fused Deposition Modelling 3D-Printed Gastro-Retentive Floating Device for Propranolol Hcl Tablets
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of Gastro-Retentive Floating Device
2.2.1. Selection of Model Drug and Polymer (Filament) for Gastro-Retentive Floating Device
2.2.2. Design of Dual-Compartment Gastro-Retentive Floating Device
2.2.3. The 3D Printing of Gastro-Retentive Floating Device
2.3. Characterization of 3D-Printed Gastro-Retentive Floating Device
2.3.1. Morphology
2.3.2. Dimensions and Weight Variation of the Gastro-Retentive Floating Devices
2.4. In Vitro Floating Ability
2.5. In Vitro Drug Release Characteristics
2.6. Drug Release Kinetics
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Gastro-Retentive Floating Device
3.2. Characterization of 3D-Printed Gastro-Retentive Floating Devices
3.3. In Vitro Floating Ability
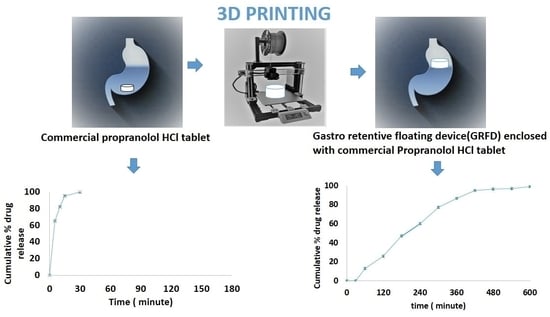
3.4. In Vitro Drug Release Characteristics
3.5. Drug Release Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci. Rep. 2017, 7, 2829. [Google Scholar] [CrossRef]
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [CrossRef]
- Tripathi, J.; Thapa, P.; Maharjan, R.; Jeong, S.H. Current State and Future Perspectives on Gastroretentive Drug Delivery Systems. Pharmaceutics 2019, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Chawla, G.; Bansal, A. A means to address regional variability in intestinal drug absorption. Pharm. Tech 2003, 27, 50–68. [Google Scholar]
- Lemieux, M.; Gosselin, P.; Mateescu, M.A. Carboxymethyl starch mucoadhesive microspheres as gastroretentive dosage form. Int. J. Pharm. 2015, 496, 497–508. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Li, S.; Li, Q.; Zhang, W.; Ye, T.; Yang, X.; Pan, W. A novel gastro-floating multiparticulate system for dipyridamole (DIP) based on a porous and low-density matrix core: In vitro and in vivo evaluation. Int. J. Pharm. 2014, 461, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Gao, B.; Ma, L.; Lian, J.; Deng, L.; Chen, J. Innovative intragastric ascaridole floating tablets: Development, optimization, and in vitro-in vivo evaluation. Int. J. Pharm. 2015, 496, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.Q.; Feng, X.; Morott, J.T.; Pimparade, M.B.; Tiwari, R.V.; Zhang, F.; Repka, M.A. A novel floating controlled release drug delivery system prepared by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2016, 98, 108–121. [Google Scholar] [CrossRef]
- Mandal, U.K.; Chatterjee, B.; Senjoti, F.G. Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian J. Pharm. Sci. 2016, 11, 575–584. [Google Scholar]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Gastroretentive drug delivery systems. Expert. Opin. Drug Deliv. 2006, 3, 217–233. [Google Scholar] [CrossRef]
- Bellinger, A.M.; Jafari, M.; Grant, T.M.; Zhang, S.; Slater, H.C.; Wenger, E.A.; Mo, S.; Lee, Y.L.; Mazdiyasni, H.; Kogan, L.; et al. Oral, ultra-long-lasting drug delivery: Application toward malaria elimination goals. Sci. Transl. Med. 2016, 8, 365ra157. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol. 2006, 6, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Madurawe, R.D.; Moore, C.M.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.A.; Ahmed, M.M.; Mohammed, A.A.; Ahmad, J. 3D Printed Pharmaceutical Systems for Personalized Treatment in Metabolic Syndrome. Pharmaceutics 2023, 15, 1152. [Google Scholar] [CrossRef]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J.; Kotta, S. 3D Printing in medicine: Technology overview and drug delivery applications. Ann. 3D Print. Med. 2021, 4, 100037. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Optimization of semisolid extrusion (pressure-assisted microsyringe)-based 3D printing process for advanced drug delivery application. Ann. 3D Print. Med. 2021, 2, 100008. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J. Extrusion-Based 3D Printing for Pharmaceuticals: Contemporary Research and Applications. Curr. Pharm. Des. 2018, 24, 4991–5008. [Google Scholar] [PubMed]
- Chen, D.; Xu, X.Y.; Li, R.; Zang, G.A.; Zhang, Y.; Wang, M.R.; Xiong, M.F.; Xu, J.R.; Wang, T.; Fu, H.; et al. Preparation and In vitro Evaluation of FDM 3D-Printed Ellipsoid-Shaped Gastric Floating Tablets with Low Infill Percentages. AAPS PharmSciTech 2019, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Ilyés, K.; Balogh, A.; Casian, T.; Igricz, T.; Borbás, E.; Démuth, B.; Vass, P.; Menyhárt, L.; Kovács, N.K.; Marosi, G.; et al. 3D floating tablets: Appropriate 3D design from the perspective of different in vitro dissolution testing methodologies. Int. J. Pharm. 2019, 567, 118433. [Google Scholar] [CrossRef]
- Qian, H.; Chen, D.; Xu, X.; Li, R.; Yan, G.; Fan, T. FDM 3D-Printed Sustained-Release Gastric-Floating Verapamil Hydrochloride Formulations with Cylinder, Capsule and Hemisphere Shapes, and Low Infill Percentage. Pharmaceutics 2022, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, W.; Niu, R.; Li, Q.; Hu, C.; Jiang, S. 3D Printed Intragastric Floating and Sustained-Release Tablets with Air Chambers. J. Pharm. Sci. 2022, 111, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.Q.; Zhang, J.; Nyavanandi, D.; Bandari, S.; Repka, M.A. Hot melt extrusion paired fused deposition modeling 3D printing to develop hydroxypropyl cellulose based floating tablets of cinnarizine. Carbohydr. Polym. 2020, 246, 116519. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Park, J.B.; Sohn, D.H.; Lee, S. Customized Novel Design of 3D Printed Pregabalin Tablets for Intra-Gastric Floating and Controlled Release Using Fused Deposition Modeling. Pharmaceutics 2019, 11, 564. [Google Scholar] [CrossRef]
- Jeong, H.M.; Weon, K.Y.; Shin, B.S.; Shin, S. 3D-Printed Gastroretentive Sustained Release Drug Delivery System by Applying Design of Experiment Approach. Molecules 2020, 25, 2330. [Google Scholar] [CrossRef]
- Charoenying, T.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Akkaramongkolporn, P.; Opanasopit, P. Fabrication of floating capsule-in-3D-printed devices as gastro-retentive delivery systems of amoxicillin. J. Drug Deliv. Sci. Technol. 2020, 55, 101393. [Google Scholar] [CrossRef]
- Fu, J.; Yin, H.; Yu, X.; Xie, C.; Jiang, H.; Jin, Y.; Sheng, F. Combination of 3D printing technologies and compressed tablets for preparation of riboflavin floating tablet-in-device (TiD) systems. Int. J. Pharm. 2018, 549, 370–379. [Google Scholar] [CrossRef]
- Shin, S.; Kim, T.H.; Jeong, S.W.; Chung, S.E.; Lee, D.Y.; Kim, D.H.; Shin, B.S. Development of a gastroretentive delivery system for acyclovir by 3D printing technology and its in vivo pharmacokinetic evaluation in Beagle dogs. PLoS ONE 2019, 14, e0216875. [Google Scholar] [CrossRef]
- Mora-Castaño, G.; Millán-Jiménez, M.; Caraballo, I. Hydrophilic High Drug-Loaded 3D Printed Gastroretentive System with Robust Release Kinetics. Pharmaceutics 2023, 15, 842. [Google Scholar] [CrossRef]
- Khizer, Z.; Akram, M.R.; Tahir, M.A.; Liu, W.; Lou, S.; Conway, B.R.; Ghori, M.U. Personalised 3D-Printed Mucoadhesive Gastroretentive Hydrophilic Matrices for Managing Overactive Bladder (OAB). Pharmaceuticals 2023, 16, 372. [Google Scholar] [CrossRef]
- Windolf, H.; Chamberlain, R.; Breitkreutz, J.; Quodbach, J. 3D Printed Mini-Floating-Polypill for Parkinson’s Disease: Combination of Levodopa, Benserazide, and Pramipexole in Various Dosing for Personalized Therapy. Pharmaceutics 2022, 14, 931. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, J.; Zhang, K.; Huang, D.; Huang, S.; Xie, Q.; Yang, F.; Huang, J.; Fang, D.; Huang, Z.; et al. Preparation of clarithromycin floating core-shell systems (CSS) using multi-nozzle semisolid extrusion-based 3D printing. Int. J. Pharm. 2021, 605, 120837. [Google Scholar] [CrossRef]
- Wen, H.; He, B.; Wang, H.; Chen, F.; Li, P.; Cui, M.; Li, Q.; Pan, W.; Yang, X. Structure-Based Gastro-Retentive and Controlled-Release Drug Delivery with Novel 3D Printing. AAPS PharmSciTech 2019, 20, 68. [Google Scholar] [CrossRef]
- Chen, P.; Luo, H.; Huang, S.; Liu, J.; Lin, M.; Yang, F.; Ban, J.; Huang, Z.; Lu, Z.; Xie, Q.; et al. Preparation of High-Drug-Loaded Clarithromycin Gastric-Floating Sustained-Release Tablets Using 3D Printing. AAPS PharmSciTech 2021, 22, 131. [Google Scholar] [CrossRef]
- Falcone, G.; Real, J.P.; Palma, S.D.; Aquino, R.P.; Del Gaudio, P.; Garofalo, E.; Russo, P. Floating Ricobendazole Delivery Systems: A 3D Printing Method by Co-Extrusion of Sodium Alginate and Calcium Chloride. Int. J. Mol. Sci. 2022, 23, 1280. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Kim, D.W. Fabrication of Gastro-Floating Famotidine Tablets: Hydroxypropyl Methylcellulose-Based Semisolid Extrusion 3D Printing. Pharmaceutics 2023, 15, 316. [Google Scholar] [CrossRef]
- Lin, X.; Fu, H.; Hou, Z.; Si, Y.; Shan, W.; Yang, Y. Three-dimensional printing of gastro-floating tablets using polyethylene glycol diacrylate-based photocurable printing material. Int. J. Pharm. 2021, 603, 120674. [Google Scholar] [CrossRef]
- Li, P.; Zhang, S.; Sun, W.; Cui, M.; Wen, H.; Li, Q.; Pan, W.; Yang, X. Flexibility of 3D Extruded Printing for a Novel Controlled-Release Puerarin Gastric Floating Tablet: Design of Internal Structure. AAPS PharmSciTech 2019, 20, 236. [Google Scholar] [CrossRef]
- Jagdale, S.C.; Agavekar, A.J.; Pandya, S.V.; Kuchekar, B.S.; Chabukswar, A.R. Formulation and evaluation of gastroretentive drug delivery system of propranolol hydrochloride. AAPS PharmSciTech 2009, 10, 1071–1079. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Saleh, E. Development of a 3D Printed Coating Shell to Control the Drug Release of Encapsulated Immediate-Release Tablets. Polymers 2020, 12, 1395. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Umadevi, S.; Vaghani, S. Floating matrix dosage form for propranolol hydrochloride based on gas formation technique: Development and in vitro evaluation. Sci. Pharm. 2010, 78, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Tagami, T.; Fukushige, K.; Ogawa, E.; Hayashi, N.; Ozeki, T. 3D Printing Factors Important for the Fabrication of Polyvinylalcohol Filament-Based Tablets. Biol. Pharm. Bull. 2017, 40, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, H.; Jia, D.; Guan, X.; Pan, H.; Yang, Y.; Yu, S.; Zhu, Z.; Xiang, R.; Pan, W. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. Int. J. Pharm. 2017, 525, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Korelidou, A.; Domínguez-Robles, J.; Magill, E.R.; Eleftheriadou, M.; Cornelius, V.A.; Donnelly, R.F.; Margariti, A.; Larrañeta, E. 3D-printed reservoir-type implants containing poly (lactic acid)/poly (caprolactone) porous membranes for sustained drug delivery. Biomater. Adv. 2022, 139, 213024. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, J.; Ervasti, T.; Laitinen, R. Production and characterization of glibenclamide incorporated PLA filaments for 3D printing by fused deposition modeling. J. Drug Deliv. Sci. Technol. 2022, 77, 103843. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef]
- Smith, D.M.; Kapoor, Y.; Klinzing, G.R.; Procopio, A.T. Pharmaceutical 3D printing: Design and qualification of a single step print and fill capsule. Int. J. Pharm. 2018, 544, 21–30. [Google Scholar] [CrossRef]
- Chareonying, T.; Opanasopit, P.; Rojanarata, T.; Akkaramongkolporn, P.; Patrojanasophon, P. Effects of thermal crosslinking on the properties and release profiles of three-dimensional (3D)-printed poly vinyl alcohol (PVA) tablets. Key Eng. Mater. 2020, 859, 258–264. [Google Scholar] [CrossRef]
- Charoenying, T.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Akkaramongkolporn, P.; Opanasopit, P. Three-dimensional (3D)-printed devices composed of hydrophilic cap and hydrophobic body for improving buoyancy and gastric retention of domperidone tablets. Eur. J. Pharm. Sci. 2020, 155, 105555. [Google Scholar] [CrossRef] [PubMed]
- Palekar, S.; Nukala, P.K.; Mishra, S.M.; Kipping, T.; Patel, K. Application of 3D printing technology and quality by design approach for development of age-appropriate pediatric formulation of baclofen. Int. J. Pharm. 2019, 556, 106–116. [Google Scholar] [CrossRef]
- Aho, J.; Bøtker, J.P.; Genina, N.; Edinger, M.; Arnfast, L.; Rantanen, J. Roadmap to 3D-Printed Oral Pharmaceutical Dosage Forms: Feedstock Filament Properties and Characterization for Fused Deposition Modeling. J. Pharm. Sci. 2019, 108, 26–35. [Google Scholar] [CrossRef]
- Arafat, B.; Wojsz, M.; Isreb, A.; Forbes, R.T.; Isreb, M.; Ahmed, W.; Arafat, T.; Alhnan, M.A. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur. J. Pharm. Sci. 2018, 118, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Srikanth, M.V.; Rao, N.S.; Sunil, S.A.; Ram, B.J.; Kolapalli, V.R.M. Statistical design and evaluation of a propranolol HCl gastric floating tablet. Acta Pharm. Sin. B 2012, 2, 60–69. [Google Scholar] [CrossRef]
- Huanbutta, K.; Sangnim, T. Design and development of zero-order drug release gastroretentive floating tablets fabricated by 3D printing technology. J. Drug Deliv. Sci. Technol. 2019, 52, 831–837. [Google Scholar] [CrossRef]
- Smith, G.; Pedge, N.; Khan, K.A.; Bukhari, N.I.; Hussain, A.; Ermolina, I. Solubility and dissolution rate enhancement of ibuprofen by co-milling with polymeric excipients. Eur. J. Pharm. Sci. 2018, 123, 395–403. [Google Scholar]
- Singpanna, K.; Charoenying, T.; Patrojanasophon, P.; Rojanarata, T.; Sukma, M.; Opanasopit, P. Fabrication of a Floating Device of Domperidone Tablets Using 3D-Printing Technologies. Key Eng. Mater. 2020, 859, 289–294. [Google Scholar] [CrossRef]
- Charoenying, T.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Akkaramongkolporn, P.; Opanasopit, P. Design and Optimization of 3D-Printed Gastroretentive Floating Devices by Central Composite Design. AAPS PharmSciTech 2021, 22, 197. [Google Scholar] [CrossRef]
- Reddy Dumpa, N.; Bandari, S.; Repka, M.A. Novel Gastroretentive Floating Pulsatile Drug Delivery System Produced via Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing. Pharmaceutics 2020, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Strübing, S.; Metz, H.; Mäder, K. Characterization of poly(vinyl acetate) based floating matrix tablets. J. Control. Release 2008, 126, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Meka, V.S.; Songa, A.S.; Nali, S.R.; Battu, J.R.; Kolapalli, V.R. Design and in vitro evaluation of effervescent gastric floating drug delivery systems of propanolol HCl. Investig. Clin. 2012, 53, 60–70. [Google Scholar]
- Qi, X.; Ren, Y.; Wang, X. New advances in the biodegradation of Poly(lactic) acid. Int. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- Bardonnet, P.L.; Faivre, V.; Pugh, W.J.; Piffaretti, J.C.; Falson, F. Gastroretentive dosage forms: Overview and special case of Helicobacter pylori. J. Control. Release 2006, 111, 1–18. [Google Scholar] [CrossRef]
- Prinderre, P.; Sauzet, C.; Fuxen, C. Advances in gastro retentive drug-delivery systems. Expert. Opin. Drug Deliv. 2011, 8, 1189–1203. [Google Scholar] [CrossRef]






| Slicing Parameters | Printing Parameters | ||
|---|---|---|---|
| Parameter | Settings | Parameter | Settings |
| Layer height | 0.18 mm | Filament used—PVA | |
| First layer height | 0.27 mm | Nozzle diameter | 0.4 mm |
| Base print speed | 60 mm/s | Filament diameter | 1.75 mm |
| Print speed | |||
| Travel speed | 100 mm/s | Printing temperature | 200 °C |
| Shell count | 2 | Bed temperature | 50 °C |
| Shell thickness | 0.8 mm | Filament used—PLA | |
| Infill density | 15% | Nozzle diameter | 0.4 mm |
| Raft | No raft | Filament diameter | 1.75 mm |
| Brim | No brim | Printing temperature | 210 °C |
| Bottom solid layers | 3 | Bed temperature | 40 °C |
| GRFDs | Filament Used | Opening Size (Diameter in mm) |
|---|---|---|
| GRFD1 | PVA | 1 mm |
| GRFD2 | PVA | 2 mm |
| GRFD3 | PVA | 3 mm |
| GRFD4 | PVA | 4 mm |
| GRFD5 | PLA | 1 mm |
| GRFD6 | PLA | 2 mm |
| GRFD7 | PLA | 3 mm |
| GRFD8 | PLA | 4 mm |
| GRFDs |
Average Diameter (mm) ± SD |
Average Thickness (mm) ± SD | Average Opening Size (mm) ± SD | Average Weight (mg) ± SD | Deviation in Weight (RSD) |
|---|---|---|---|---|---|
| GRFD1 | 12.00 ± 0.02 | 5.04 ± 0.02 | 0.11 ± 0.01 | 432.24 ± 4.24 | 0.98% |
| GRFD2 | 12.02 ± 0.01 | 5.04 ± 0.01 | 0.20 ± 0.02 | 424.30 ± 7.83 | 1.84% |
| GRFD3 | 12.00 ± 0.02 | 5.03 ± 0.02 | 0.33 ± 0.02 | 420.36 ± 8.21 | 1.95% |
| GRFD4 | 12.00 ± 0.02 | 5.05 ± 0.02 | 0.41 ± 0.02 | 418.52 ± 4.36 | 1.04% |
| GRFD5 | 12.03 ± 0.01 | 5.03 ± 0.03 | 0.10 ± 0.00 | 402.42 ± 10.12 | 2.51% |
| GRFD6 | 12.02 ± 0.02 | 5.02 ± 0.02 | 0.22 ± 0.08 | 390.12 ± 5.54 | 1.42% |
| GRFD7 | 12.01 ± 0.01 | 5.05 ± 0.01 | 0.31 ± 0.02 | 382.36 ± 9.76 | 2.55% |
| GRFD8 | 12.00 ± 0.01 | 5.04 ± 0.03 | 0.42 ± 0.06 | 380.46 ± 9.34 | 2.45% |
| Density (mg/mm3) | In Vitro Floating Behavior (n = 3) | ||
|---|---|---|---|
| Total Floating Time (h) | Floating Lag Time (min) | ||
| Propranolol HCl tablet | 0 min | Sunk | |
| GRFD1 | 0.76 ± 0.01 | 3 h 42 min 34 s ± 4 min 20 s | 0 min |
| GRFD2 | 0.75 ± 0.01 | 3 h 42 min 12 s ± 6 min 16 s | 0 min |
| GRFD3 | 0.74 ± 0.02 | 3 h 39 min 54 s ± 7 min 42 s | 0 min |
| GRFD4 | 0.74 ± 0.01 | 3 h 33 min 37 s ± 4 min 37 s | 0 min |
| GRFD5 | 0.71 ± 0.01 | >24 h | 0 min |
| GRFD6 | 0.69 ± 0.01 | >24 h | 0 min |
| GRFD7 | 0.67 ± 0.02 | >24 h | 0 min |
| GRFD8 | 0.67 ± 0.01 | >24 h | 0 min |
| GRFDs | Zero-Order (r2) | First-Order (r2) | Higuchi (r2) | Korsmeyer–Peppas | |
|---|---|---|---|---|---|
| (r2) | n Value | ||||
| GRFD1 | 0.9171 | 0.9028 | 0.7977 | 0.5163 | 0.86 |
| GRFD2 | 0.9093 | 0.9057 | 0.7971 | 0.5183 | 0.88 |
| GRFD3 | 0.8850 | 0.9218 | 0.8579 | 0.3988 | 0.82 |
| GRFD4 | 0.8199 | 0.9392 | 0.8487 | 0.3565 | 0.82 |
| GRFD5 | 0.9816 | 0.9567 | 0.8606 | 0.8916 | 0.66 |
| GRFD6 | 0.9832 | 0.9564 | 0.8635 | 0.8938 | 0.70 |
| GRFD7 | 0.9449 | 0.9497 | 0.9369 | 0.8258 | 0.89 |
| GRFD8 | 0.9234 | 0.9623 | 0.9466 | 0.7954 | 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, A.A.; Mohammed, A.A.; Fatima, F.; Ahmed, M.M. Fused Deposition Modelling 3D-Printed Gastro-Retentive Floating Device for Propranolol Hcl Tablets. Polymers 2023, 15, 3554. https://doi.org/10.3390/polym15173554
Alqahtani AA, Mohammed AA, Fatima F, Ahmed MM. Fused Deposition Modelling 3D-Printed Gastro-Retentive Floating Device for Propranolol Hcl Tablets. Polymers. 2023; 15(17):3554. https://doi.org/10.3390/polym15173554
Chicago/Turabian StyleAlqahtani, Abdulsalam A., Abdul Aleem Mohammed, Farhat Fatima, and Mohammed Muqtader Ahmed. 2023. "Fused Deposition Modelling 3D-Printed Gastro-Retentive Floating Device for Propranolol Hcl Tablets" Polymers 15, no. 17: 3554. https://doi.org/10.3390/polym15173554