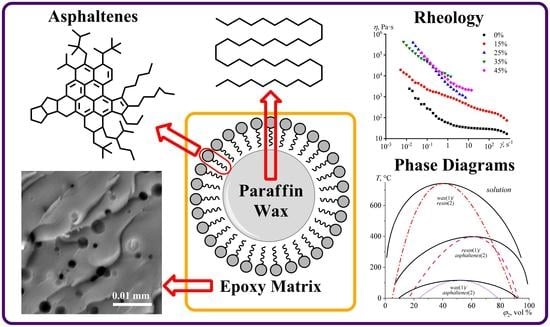
Epoxy Phase-Change Materials Based on Paraffin Wax Stabilized by Asphaltenes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Phase State of Asphaltenes/Paraffin Wax/Epoxy Resin Blends
3.2. Rheological Properties of Uncured Blends
3.3. Curing of the Blend
3.4. Thermophysical and Morphological Features of Cured Blends
4. Conclusions
- Epoxy resin and paraffin wax are poorly miscible even during high-temperature curing at 180 °C, which leads to a two-phase curing mixture (a wax-in-resin emulsion). In turn, asphaltenes dissolve in both phases at high temperatures, entering them and stabilizing the molten wax droplets.
- Uncured blends are non-Newtonian fluids with yield stress at 25 °C due to a structural network from hardener and paraffin wax particles. The network disappears at heating due to wax melting, causing the blends to become Newtonian fluids.
- The paraffin wax increases the viscosity of the epoxy resin up to 10 times but has no pronounced effect on the cross-linking rate and its completeness. At the same time, the asphaltenes may be involved in a chemical reaction with the epoxy resin, increasing the thermal effect of curing.
- Although the paraffin wax does not plasticize the cured epoxy matrix, it does not fully crystallize due to its small dispersed size.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tvaronavičienė, M.; Baublys, J.; Raudeliūnienė, J.; Jatautaitė, D. Global Energy Consumption Peculiarities and Energy Sources. In Energy Transformation Towards Sustainability; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–49. ISBN 978-0-12-817688-7. [Google Scholar]
- Ahmad, T.; Zhang, D. A Critical Review of Comparative Global Historical Energy Consumption and Future Demand: The Story Told so Far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V. Bio-Oil: Production, Modification, and Application. Chem. Technol. Fuels Oils 2022, 58, 29–44. [Google Scholar] [CrossRef]
- Anisur, M.R.; Mahfuz, M.H.; Kibria, M.A.; Saidur, R.; Metselaar, I.H.S.C.; Mahlia, T.M.I. Curbing Global Warming with Phase Change Materials for Energy Storage. Renew. Sustain. Energy Rev. 2013, 18, 23–30. [Google Scholar] [CrossRef]
- Da Cunha, S.R.L.; de Aguiar, J.L.B. Phase Change Materials and Energy Efficiency of Buildings: A Review of Knowledge. J. Energy Storage 2020, 27, 101083. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.; Chan, C. Application of Phase Change Materials for Thermal Energy Storage in Concentrated Solar Thermal Power Plants: A Review to Recent Developments. Appl. Energy 2015, 160, 286–307. [Google Scholar] [CrossRef]
- Nie, B.; Palacios, A.; Zou, B.; Liu, J.; Zhang, T.; Li, Y. Review on Phase Change Materials for Cold Thermal Energy Storage Applications. Renew. Sustain. Energy Rev. 2020, 134, 110340. [Google Scholar] [CrossRef]
- Iqbal, K.; Khan, A.; Sun, D.; Ashraf, M.; Rehman, A.; Safdar, F.; Basit, A.; Maqsood, H.S. Phase Change Materials, Their Synthesis and Application in Textiles—A Review. J. Text. Inst. 2019, 110, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Goel, V.; Saxena, A.; Kumar, M.; Thakur, A.; Sharma, A.; Bianco, V. Potential of Phase Change Materials and Their Effective Use in Solar Thermal Applications: A Critical Review. Appl. Therm. Eng. 2023, 219, 119417. [Google Scholar] [CrossRef]
- Cai, R.; Sun, Z.; Yu, H.; Meng, E.; Wang, J.; Dai, M. Review on Optimization of Phase Change Parameters in Phase Change Material Building Envelopes. J. Build. Eng. 2021, 35, 101979. [Google Scholar] [CrossRef]
- Song, M.; Niu, F.; Mao, N.; Hu, Y.; Deng, S. Review on Building Energy Performance Improvement Using Phase Change Materials. Energy Build. 2018, 158, 776–793. [Google Scholar] [CrossRef]
- Mahmoud, M.; Ramadan, M.; Olabi, A.-G.; Pullen, K.; Naher, S. A Review of Mechanical Energy Storage Systems Combined with Wind and Solar Applications. Energy Convers. Manag. 2020, 210, 112670. [Google Scholar] [CrossRef]
- Kostyuk, A.V.; Smirnova, N.M.; Ilyin, S.O. Two-Functional Phase-Change Pressure-Sensitive Adhesives Based on Polyisobutylene Matrix Filled with Paraffin Wax. J. Energy Storage 2022, 52, 104797. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Bolivar Osorio, F.J.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent Developments in Phase Change Materials for Energy Storage Applications: A Review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Matuszek, K.; Kar, M.; Pringle, J.M.; MacFarlane, D.R. Phase Change Materials for Renewable Energy Storage at Intermediate Temperatures. Chem. Rev. 2023, 123, 491–514. [Google Scholar] [CrossRef] [PubMed]
- Kalnæs, S.E.; Jelle, B.P. Phase Change Materials and Products for Building Applications: A State-of-the-Art Review and Future Research Opportunities. Energy Build. 2015, 94, 150–176. [Google Scholar] [CrossRef] [Green Version]
- Gobinath, G.; Rama, M.; Vetri Selvi, P. An Overview of Phase Change Materials and Their Applications in Construction Industry: Review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Chopra, K.; Sharma, R.K.; Pandey, A.K.; Tyagi, S.K.; Ahmad, M.S.; Sarı, A.; Kothari, R. A Comprehensive Review on Phase Change Materials for Heat Storage Applications: Development, Characterization, Thermal and Chemical Stability. Sol. Energy Mater. Sol. Cells 2022, 234, 111392. [Google Scholar] [CrossRef]
- Sun, M.; Liu, T.; Sha, H.; Li, M.; Liu, T.; Wang, X.; Chen, G.; Wang, J.; Jiang, D. A Review on Thermal Energy Storage with Eutectic Phase Change Materials: Fundamentals and Applications. J. Energy Storage 2023, 68, 107713. [Google Scholar] [CrossRef]
- Raoux, S. Phase Change Materials. Annu. Rev. Mater. Res. 2009, 39, 25–48. [Google Scholar] [CrossRef]
- Demirbas, M.F. Thermal Energy Storage and Phase Change Materials: An Overview. Energy Sources Part B Econ. Plan. Policy 2006, 1, 85–95. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on Thermal Energy Storage with Phase Change Materials and Applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.; Moqeet Hai, A.; Zhang, S.; Tang, B. Shape-Stabilization Micromechanisms of Form-Stable Phase Change Materials-A Review. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107047. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Zhang, J.; Lin, L.; Shi, J. A Review of Composite Phase Change Materials Based on Biomass Materials. Polymers 2022, 14, 4089. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Imran, M.; Zhang, Y.; Jia, Z.; Lu, X.; Lu, R.; Tang, B. Form-Stable Phase Change Material with Wood-Based Materials as Support. Polymers 2023, 15, 942. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Review on Clay Mineral-Based Form-Stable Phase Change Materials: Preparation, Characterization and Applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Encapsulation Techniques for Organic Phase Change Materials as Thermal Energy Storage Medium: A Review. Sol. Energy Mater. Sol. Cells 2015, 143, 78–98. [Google Scholar] [CrossRef]
- Wu, C.-B.; Wu, G.; Yang, X.; Liu, Y.-J.; Liang, T.; Fu, W.-F.; Wang, M.; Chen, H.-Z. Preparation of Microencapsulated Medium Temperature Phase Change Material of Tris(Hydroxymethyl)Methyl Aminomethane@SiO2 with Excellent Cycling Performance. Appl. Energy 2015, 154, 361–368. [Google Scholar] [CrossRef]
- Wang, L.; Kong, X.; Ren, J.; Fan, M.; Li, H. Novel Hybrid Composite Phase Change Materials with High Thermal Performance Based on Aluminium Nitride and Nanocapsules. Energy 2022, 238, 121775. [Google Scholar] [CrossRef]
- Ma, J.; Ma, T.; Cheng, J.; Zhang, J. Polymer Encapsulation Strategy toward 3D Printable, Sustainable, and Reliable Form-Stable Phase Change Materials for Advanced Thermal Energy Storage. ACS Appl. Mater. Interfaces 2022, 14, 4251–4264. [Google Scholar] [CrossRef]
- Li, W.; Mei, D.; Wang, J.; Wu, H.; Wen, S. Preparation of Microencapsulated Phase Change Materials from Sulfonated Graphene Stabilized Pickering Emulsion. Polymers 2023, 15, 2441. [Google Scholar] [CrossRef]
- Huang, Y.; Stonehouse, A.; Abeykoon, C. Encapsulation Methods for Phase Change Materials—A Critical Review. Int. J. Heat Mass Transf. 2023, 200, 123458. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel Strategies and Supporting Materials Applied to Shape-Stabilize Organic Phase Change Materials for Thermal Energy Storage–A Review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Gencel, O.; Nodehi, M.; Hekimoğlu, G.; Ustaoğlu, A.; Sarı, A.; Kaplan, G.; Bayraktar, O.Y.; Sutcu, M.; Ozbakkaloglu, T. Foam Concrete Produced with Recycled Concrete Powder and Phase Change Materials. Sustainability 2022, 14, 7458. [Google Scholar] [CrossRef]
- Aiswarya, V.; Das, S. Magnetized Graphene Oxide—Modified Microencapsulated Phase Change Material for Enhanced Heat Transfer Performance with Reduced Leakage. Therm. Sci. Eng. Prog. 2023, 41, 101807. [Google Scholar] [CrossRef]
- Nistor, C.L.; Gifu, I.C.; Anghel, E.M.; Ianchis, R.; Cirstea, C.-D.; Nicolae, C.A.; Gabor, A.R.; Atkinson, I.; Petcu, C. Novel PEG6000–Silica-MWCNTs Shape-Stabilized Composite Phase-Change Materials (SsCPCMs) for Thermal-Energy Storage. Polymers 2023, 15, 3022. [Google Scholar] [CrossRef]
- Yu, C.; Song, Y. Modified Supporting Materials to Fabricate Form Stable Phase Change Material with High Thermal Energy Storage. Molecules 2023, 28, 1309. [Google Scholar] [CrossRef]
- Huang, X.; Alva, G.; Jia, Y.; Fang, G. Morphological Characterization and Applications of Phase Change Materials in Thermal Energy Storage: A Review. Renew. Sustain. Energy Rev. 2017, 72, 128–145. [Google Scholar] [CrossRef]
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal Conductivity Enhancement on Phase Change Materials for Thermal Energy Storage: A Review. Energy Storage Mater. 2020, 25, 251–295. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Makarova, V.V.; Ilyin, S.O. Hydrophobic Nanosilica-Stabilized Graphite Particles for Improving Thermal Conductivity of Paraffin Wax-Based Phase-Change Materials. J. Energy Storage 2021, 36, 102417. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase Change Materials for Thermal Energy Storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Xu, Y.; Chen, G.; Lin, F.; Ding, H. Preparation and Thermal Properties of Shape-Stabilized Composite Phase Change Materials Based on Paraffin Wax and Carbon Foam. Polymer 2021, 237, 124361. [Google Scholar] [CrossRef]
- Weingrill, H.M.; Resch-Fauster, K.; Zauner, C. Applicability of Polymeric Materials as Phase Change Materials. Macromol. Mater. Eng. 2018, 303, 1800355. [Google Scholar] [CrossRef]
- Vasilyev, G.; Koifman, N.; Shuster, M.; Gishvoliner, M.; Cohen, Y.; Zussman, E. Synergistic Effect of Two Organogelators for the Creation of Bio-Based, Shape-Stable Phase-Change Materials. Langmuir 2020, 36, 15572–15582. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lu, S.; Kong, X.; Liu, S.; Li, Y. Form-Stable Phase Change Materials Based on Eutectic Mixture of Tetradecanol and Fatty Acids for Building Energy Storage: Preparation and Performance Analysis. Materials 2013, 6, 4758–4775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Liu, Y.; He, R.; Wang, Q. Preparation and Characterization of Polyethylene Glycol-based Form-stable Phase Change Materials Supported by Poly (Vinyl Formal) Foams. J Appl. Polym. Sci 2022, 139, e52625. [Google Scholar] [CrossRef]
- Inaba, H.; Tu, P. Evaluation of Thermophysical Characteristics on Shape-Stabilized Paraffin as a Solid-Liquid Phase Change Material. Heat Mass Transf. 1997, 32, 307–312. [Google Scholar] [CrossRef]
- Xiao, M.; Feng, B.; Gong, K. Preparation and Performance of Shape Stabilized Phase Change Thermal Storage Materials with High Thermal Conductivity. Energy Convers. Manag. 2002, 43, 103–108. [Google Scholar] [CrossRef]
- Vinogradov, G.V. Ultimate Regimes of Deformation of Linear Flexible Chain Fluid Polymers. Polymer 1977, 18, 1275–1285. [Google Scholar] [CrossRef]
- Wang, T.; Wang, C.; Chen, K.; Liu, X.; Huang, Z. Preparation, Thermal, and Mechanical Properties of Polyethylene Glycol/Expoxy Resin Composites as Form-stable Phase Change Materials. Polym. Eng. Sci. 2022, 62, 520–529. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, X.; Zhang, J.; Cheng, J.; Lian, Q. Relationship between Cross-Linking Network Structure and Phase Change Performances toward Multifunctional Epoxy/Bio-Based Wax Form-Stable Phase Change Materials. Chem. Eng. J. 2023, 454, 140221. [Google Scholar] [CrossRef]
- Wang, X.; Ma, B.; Wei, K.; Zhang, W. Thermal Stability and Mechanical Properties of Epoxy Resin/Microcapsule Composite Phase Change Materials. Constr. Build. Mater. 2021, 312, 125392. [Google Scholar] [CrossRef]
- Cheng, P.; Wei, K.; Shi, W.; Shi, J.; Wang, S.; Ma, B. Preparation and Performance Analysis of Phase Change Microcapsule/Epoxy Resin Composite Phase Change Material. J. Energy Storage 2022, 47, 103581. [Google Scholar] [CrossRef]
- Babak, V.G.; Stébé, M.-J. Highly Concentrated Emulsions: Physicochemical Principles of Formulation. J. Dispers. Sci. Technol. 2002, 23, 1–22. [Google Scholar] [CrossRef]
- Cohen-Addad, S.; Höhler, R. Rheology of Foams and Highly Concentrated Emulsions. Curr. Opin. Colloid Interface Sci. 2014, 19, 536–548. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions Stabilized with Solid Nanoparticles: Pickering Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Wang, Z.; Situ, W.; Li, X.; Zhang, G.; Huang, Z.; Yuan, W.; Yang, C.; Yang, C. Novel Shape Stabilized Phase Change Material Based on Epoxy Matrix with Ultrahigh Cycle Life for Thermal Energy Storage. Appl. Therm. Eng. 2017, 123, 1006–1012. [Google Scholar] [CrossRef]
- Nushtaeva, A.V. Superstabilization of Emulsions by Solid Particles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 283–287. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Ilyin, S.O. Morphology and Rheology of Heavy Crude Oil/Water Emulsions Stabilized by Microfibrillated Cellulose. Energy Fuels 2021, 35, 6527–6540. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef] [Green Version]
- Mironova, M.V.; Ilyin, S.O. Effect of Silica and Clay Minerals on Rheology of Heavy Crude Oil Emulsions. Fuel 2018, 232, 290–298. [Google Scholar] [CrossRef]
- Makarova, V.V.; Gorbacheva, S.N.; Antonov, S.V.; Ilyin, S.O. On the Possibility of a Radical Increase in Thermal Conductivity by Dispersed Particles. Russ. J. Appl. Chem. 2020, 93, 1796–1814. [Google Scholar] [CrossRef]
- Umar, A.A.; Saaid, I.B.M.; Sulaimon, A.A.; Pilus, R.B.M. A Review of Petroleum Emulsions and Recent Progress on Water-in-Crude Oil Emulsions Stabilized by Natural Surfactants and Solids. J. Pet. Sci. Eng. 2018, 165, 673–690. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Ilyin, S.O. Structure, Rheology and Possible Application of Water-in-Oil Emulsions Stabilized by Asphaltenes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126442. [Google Scholar] [CrossRef]
- Ilyin, S.; Arinina, M.; Polyakova, M.; Bondarenko, G.; Konstantinov, I.; Kulichikhin, V.; Malkin, A. Asphaltenes in Heavy Crude Oil: Designation, Precipitation, Solutions, and Effects on Viscosity. J. Pet. Sci. Eng. 2016, 147, 211–217. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostyuk, A.V.; Kostina, J.V.; Bakhtin, D.S.; Makarova, V.V.; Antonov, S.V.; Ilyin, S.O. Heavy Crude Oil Asphaltenes as a Nanofiller for Epoxy Resin. Polym. Eng. Sci. 2020, 60, 1530–1545. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostyuk, A.V.; Smirnova, N.M.; Antonov, S.V.; Ilyin, S.O. Asphaltenes as a Tackifier for Hot-melt Adhesives Based on the Styrene-isoprene-styrene Block Copolymer. Polym. Eng. Sci. 2020, 60, 2224–2234. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Melekhina, V.Y.; Kostyuk, A.V.; Smirnova, N.M. Hot-Melt and Pressure-Sensitive Adhesives Based on Styrene-Isoprene-Styrene Triblock Copolymer, Asphaltene/Resin Blend and Naphthenic Oil. Polymers 2022, 14, 4296. [Google Scholar] [CrossRef]
- Melekhina, V.Y.; Kostyuk, A.V.; Smirnova, N.M.; Ilyin, S.O. Asphaltene-Stabilized Polyisobutylene Pressure-Sensitive Adhesives for Ultraviolet Protection and Surface Bonding. Materials 2023, 16, 1209. [Google Scholar] [CrossRef]
- Ghasemirad, A.; Bala, N.; Hashemian, L. High-Temperature Performance Evaluation of Asphaltenes-Modified Asphalt Binders. Molecules 2020, 25, 3326. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Vakhin, A.V.; Mikhailova, A.N.; Petrov, S.M.; Sitnov, S.A. Road Bitumen’s Based on the Vacuum Residue of Heavy Oil and Natural Asphaltite: Part II—Physical and Mechanical Properties. Pet. Sci. Technol. 2017, 35, 1687–1691. [Google Scholar] [CrossRef]
- Kamkar, M.; Natale, G. A Review on Novel Applications of Asphaltenes: A Valuable Waste. Fuel 2021, 285, 119272. [Google Scholar] [CrossRef]
- Mullins, O.C. The Asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393–418. [Google Scholar] [CrossRef]
- Rocha, J.A.; Baydak, E.N.; Yarranton, H.W. What Fraction of the Asphaltenes Stabilizes Water-in-Bitumen Emulsions? Energy Fuels 2018, 32, 1440–1450. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Hussein, H.; Masliyah, J.H. Water-in-Hydrocarbon Emulsions Stabilized by Asphaltenes at Low Concentrations. J. Colloid Interface Sci. 2000, 228, 52–63. [Google Scholar] [CrossRef]
- Qiao, P.; Harbottle, D.; Tchoukov, P.; Masliyah, J.; Sjoblom, J.; Liu, Q.; Xu, Z. Fractionation of Asphaltenes in Understanding Their Role in Petroleum Emulsion Stability and Fouling. Energy Fuels 2017, 31, 3330–3337. [Google Scholar] [CrossRef]
- Nenningsland, A.L.; Simon, S.; Sjöblom, J. Influence of Interfacial Rheological Properties on Stability of Asphaltene-Stabilized Emulsions. J. Dispers. Sci. Technol. 2014, 35, 231–243. [Google Scholar] [CrossRef]
- Sjöblom, J.; Hemmingsen, P.V.; Kallevik, H. The Role of Asphaltenes in Stabilizing Water-in-Crude Oil Emulsions. In Asphaltenes, Heavy Oils, and Petroleomics; Mullins, O.C., Sheu, E.Y., Hammami, A., Marshall, A.G., Eds.; Springer: New York, NY, USA, 2007; pp. 549–587. ISBN 978-0-387-31734-2. [Google Scholar]
- Yadykova, A.Y.; Ilyin, S.O. Compatibility and Rheology of Bio-Oil Blends with Light and Heavy Crude Oils. Fuel 2022, 314, 122761. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Strelets, L.A.; Ilyin, S.O. Infrared Spectral Classification of Natural Bitumens for Their Rheological and Thermophysical Characterization. Molecules 2023, 28, 2065. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostina, Y.V.; Antonov, S.V.; Ilyin, S.O. Oxidative Functionalization of Asphaltenes from Heavy Crude Oil. Russ. J. Appl. Chem. 2018, 91, 1835–1840. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Ignatenko, V.Y.; Kostyuk, A.V.; Levin, I.S.; Bondarenko, G.N. Deasphalting of Heavy Crude Oil by Hexamethyldisiloxane: The Effect of a Solvent/Oil Ratio on the Structure, Composition, and Properties of Precipitated Asphaltenes. J. Pet. Sci. Eng. 2022, 208, 109329. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Ilyin, S.O. Rheological and Adhesive Properties of Nanocomposite Bitumen Binders Based on Hydrophilic or Hydrophobic Silica and Modified with Bio-Oil. Constr. Build. Mater. 2022, 342, 127946. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, X.; Zhang, G. Durability of Phase-Change-Material Module and Its Relieving Effect on Battery Deterioration during Long-Term Cycles. Appl. Therm. Eng. 2020, 179, 115747. [Google Scholar] [CrossRef]
- Rahmalina, D.; Rahman, R.A. Ismail Increasing the Rating Performance of Paraffin up to 5000 Cycles for Active Latent Heat Storage by Adding High-Density Polyethylene to Form Shape-Stabilized Phase Change Material. J. Energy Storage 2022, 46, 103762. [Google Scholar] [CrossRef]
- Sarı, A.; Hekimoğlu, G.; Tyagi, V.V. Low Cost and Eco-Friendly Wood Fiber-Based Composite Phase Change Material: Development, Characterization and Lab-Scale Thermoregulation Performance for Thermal Energy Storage. Energy 2020, 195, 116983. [Google Scholar] [CrossRef]
- Jia, W.; Wang, C.; Wang, T.; Cai, Z.; Chen, K. Preparation and Performances of Palmitic Acid/Diatomite Form-stable Composite Phase Change Materials. Int. J. Energy Res. 2020, 44, 4298–4308. [Google Scholar] [CrossRef]
- Fang, G.; Zhang, W.; Yu, M.; Meng, K.; Tan, X. Experimental Investigation of High Performance Composite Phase Change Materials Based on Sodium Acetate Trihydrate for Solar Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2022, 234, 111418. [Google Scholar] [CrossRef]
- Alkhazaleh, A.H.; Almanaseer, W.; Alkhazali, A. Experimental Investigation on Thermal Properties and Fire Performance of Lauric Acid/Diphenyl Phosphate/Expanded Perlite as a Flame Retardant Phase Change Material for Latent Heat Storage Applications. Sustain. Energy Technol. Assess. 2023, 56, 103059. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-429-12752-6. [Google Scholar]
- Brandrup, J.; Immergut, E.H. Polymer Handbook, 3rd ed.; Wiley: Hoboken, NJ, USA, 1989. [Google Scholar]
- Andersen, S.I. Flocculation Onset Titration of Petroleum Asphaltenes. Energy Fuels 1999, 13, 315–322. [Google Scholar] [CrossRef]
- Hirschberg, A.; deJong, L.N.J.; Schipper, B.A.; Meijer, J.G. Influence of Temperature and Pressure on Asphaltene Flocculation. Soc. Pet. Eng. J. 1984, 24, 283–293. [Google Scholar] [CrossRef]
- Scott, R.L. The Thermodynamics of High Polymer Solutions. IV. Phase Equilibria in the Ternary System: Polymer—Liquid 1—Liquid 2. J. Chem. Phys. 1949, 17, 268–279. [Google Scholar] [CrossRef]
- Krigbaum, W.R.; Carpenter, D.K. Phase Equilibria in Polymer–Liquid 1–Liquid 2 Systems. J. Polym. Sci. 1954, 14, 241–259. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Anokhina, T.S.; Ignatenko, V.Y.; Brantseva, T.V.; Volkov, A.V.; Antonov, S.V. Diffusion and Phase Separation at the Morphology Formation of Cellulose Membranes by Regeneration from N-Methylmorpholine N-Oxide Solutions. Cellulose 2018, 25, 2515–2530. [Google Scholar] [CrossRef]
- Minton, A.P. Simple Calculation of Phase Diagrams for Liquid–Liquid Phase Separation in Solutions of Two Macromolecular Solute Species. J. Phys. Chem. B 2020, 124, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Hoy, K.L. Solubility Parameter as a Design Parameter for Water Borne Polymers and Coatings. J. Coat. Fabr. 1989, 19, 53–67. [Google Scholar] [CrossRef]
- Barton, A.F.M. Handbook of Polymer-Liquid Interaction Parameters and Solubility Parameters, 1st ed.; Routledge: Abingdon, UK, 2018; ISBN 978-0-203-75261-6. [Google Scholar]
- Moebus, J.A.; Greenhalgh, B.R. Modeling Vapor Solubility in Semicrystalline Polyethylene. Macromol. React. Eng. 2018, 12, 1700072. [Google Scholar] [CrossRef]
- MacKnight, W.J.; Karasz, F. Polymer Blends. In Comprehensive Polymer Science; Pergamon: Oxford, UK, 1989; Volume 7, Chapter 4. [Google Scholar]
- Chung, T.-S. The Limitations of Using Flory-Huggins Equation for the States of Solutions during Asymmetric Hollow-Fiber Formation. J. Membr. Sci. 1997, 126, 19–34. [Google Scholar] [CrossRef]
- Ilyina, S.O.; Anokhina, T.S.; Ilyin, S.O. Non-Solvent- and Temperature-Induced Phase Separations of Polylaurolactam Solutions in Benzyl Alcohol as Methods for Producing Microfiltration Membranes. Colloids Interfaces 2023, 7, 10. [Google Scholar] [CrossRef]
- Utracki, L.A. On the Viscosity-concentration Dependence of Immiscible Polymer Blends. J. Rheol. 1991, 35, 1615–1637. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Polyakova, M.Y.; Kulichikhin, V.G. Phase State and Rheology of Polyisobutylene Blends with Silicone Resin. Rheol. Acta 2020, 59, 375–386. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Polyakova, M.Y.; Kulichikhin, V.G. Phase Behavior and Rheology of Miscible and Immiscible Blends of Linear and Hyperbranched Siloxane Macromolecules. Mater. Today Commun. 2020, 22, 100833. [Google Scholar] [CrossRef]
- Rehbinder, P.A. Formation of Structures in Disperse Systems. Pure Appl. Chem. 1965, 10, 337–358. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Ilyin, S.O.; Arinina, M.P.; Kulichikhin, V.G. The Rheological State of Suspensions in Varying the Surface Area of Nano-Silica Particles and Molecular Weight of the Poly(Ethylene Oxide) Matrix. Colloid Polym. Sci. 2017, 295, 555–563. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Malkin, A.Y.; Kulichikhin, V.G.; Shaulov, A.Y.; Stegno, E.V.; Berlin, A.A.; Patlazhan, S.A. Rheological Properties of Polyethylene/Metaboric Acid Thermoplastic Blends. Rheol. Acta 2014, 53, 467–475. [Google Scholar] [CrossRef]
- Robertson, C.G.; Roland, C.M. Glass Transition and Interfacial Segmental Dynamics in Polymer-Particle Composites. Rubber Chem. Technol. 2008, 81, 506–522. [Google Scholar] [CrossRef]
- Tsagaropoulos, G.; Eisenburg, A. Direct Observation of Two Glass Transitions in Silica-Filled Polymers. Implications to the Morphology of Random Ionomers. Macromolecules 1995, 28, 396–398. [Google Scholar] [CrossRef]
- Tsagaropoulos, G.; Eisenberg, A. Dynamic Mechanical Study of the Factors Affecting the Two Glass Transition Behavior of Filled Polymers. Similarities and Differences with Random Ionomers. Macromolecules 1995, 28, 6067–6077. [Google Scholar] [CrossRef]
- Robertson, C.G.; Rackaitis, M. Further Consideration of Viscoelastic Two Glass Transition Behavior of Nanoparticle-Filled Polymers. Macromolecules 2011, 44, 1177–1181. [Google Scholar] [CrossRef]
- Toyen, D.; Saenboonruang, K. Development of Paraffin and Paraffin/Bitumen Composites with Additions of B2O3 for Thermal Neutron Shielding Applications. J. Nucl. Sci. Technol. 2017, 54, 871–877. [Google Scholar] [CrossRef]
- Fenton, A.M.; Xie, R.; Aplan, M.P.; Lee, Y.; Gill, M.G.; Fair, R.; Kempe, F.; Sommer, M.; Snyder, C.R.; Gomez, E.D.; et al. Predicting the Plateau Modulus from Molecular Parameters of Conjugated Polymers. ACS Cent. Sci. 2022, 8, 268–274. [Google Scholar] [CrossRef]
- Abdalla, M.; Dean, D.; Adibempe, D.; Nyairo, E.; Robinson, P.; Thompson, G. The Effect of Interfacial Chemistry on Molecular Mobility and Morphology of Multiwalled Carbon Nanotubes Epoxy Nanocomposite. Polymer 2007, 48, 5662–5670. [Google Scholar] [CrossRef]
- Longun, J.; Iroh, J.O. Nano-Graphene/Polyimide Composites with Extremely High Rubbery Plateau Modulus. Carbon 2012, 50, 1823–1832. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kotomin, S.V. Effect of Nanoparticles and Their Anisometry on Adhesion and Strength in Hybrid Carbon-Fiber-Reinforced Epoxy Nanocomposites. J. Compos. Sci. 2023, 7, 147. [Google Scholar] [CrossRef]
- Cui, W.; You, W.; Yu, W. Mechanism of Mechanical Reinforcement for Weakly Attractive Nanocomposites in Glassy and Rubbery States. Macromolecules 2021, 54, 824–834. [Google Scholar] [CrossRef]
- Said, A.; Salah, A.; Fattah, G. Enhanced Thermo-Optical Switching of Paraffin-Wax Composite Spots under Laser Heating. Materials 2017, 10, 525. [Google Scholar] [CrossRef] [Green Version]
- Michell, R.M.; Blaszczyk-Lezak, I.; Mijangos, C.; Müller, A.J. Confinement Effects on Polymer Crystallization: From Droplets to Alumina Nanopores. Polymer 2013, 54, 4059–4077. [Google Scholar] [CrossRef]













| # | Epoxy resin, wt% | Hardener, wt% | Paraffin wax, wt% | Asphaltenes, wt% |
|---|---|---|---|---|
| 1 | 74.4 | 25.6 | 0 | 0 |
| 2 | 62.1 | 21.4 | 15 | 1.5 |
| 3 | 53.9 | 18.6 | 25 | 2.5 |
| 4 | 45.8 | 15.7 | 35 | 3.5 |
| 5 | 37.6 | 12.9 | 45 | 4.5 |
| wwax, wt% | Tons, °C | Tmax, °C | Tgel, °C | Tend, °C | ΔH, J/g | ΔHred, J/g |
|---|---|---|---|---|---|---|
| 0 | 140.3 | 186.3 | 202 | 248 | 300 | 300 |
| 15 | 143.5 | 176.6 | 199 | 253 | 294 | 352 |
| 25 | 147.3 | 182.6 | 207 | 243 | 267 | 368 |
| 35 | 146.6 | 182.6 | 198 | 231 | 195 | 318 |
| 45 | 143.8 | 180.1 | 207 | 227 | 173 | 342 |
| Standard deviation | 0.3 | 0.2 | 1 | 1 | 15 | 15 |
| wwax, wt% | Tg,G′, °C | Tg,G″, °C | Tg,tanδ, °C | G′30°C, MPa | G′250°C, MPa |
|---|---|---|---|---|---|
| 0 | 184.9 | 198.7 | 210.7 | 1960 | 28.7 |
| 15 | 192.4 | 204.0 | 214.9 | 1360 | 19.8 |
| 25 | 194.4 | 207.1 | 214.8 | 1030 | 21.5 |
| 35 | 196.7 | 206.8 | 216.7 | 904 | 12.0 |
| Standard deviation | 0.2 | 0.2 | 0.2 | 10% | 10% |
| wwax, wt% | Tg, °C | Tcr, °C | Tm, °C | ΔHcr, J/g | ΔHm, J/g | DC, % |
|---|---|---|---|---|---|---|
| 0 | 178.2 | - | - | - | - | - |
| 15 | 196.6 | 47.6 | 57.3 | 7.8 | 8.4 | 28.7 |
| 25 | 189.8 | 46.1 | 54.0 | 4.4 | 7.4 | 12.5 |
| 35 | 193.9 | 49.1 | 59.6 | 13.6 | 13.1 | 20.2 |
| 45 | 191.7 | 47.4 | 58.0 | 12.0 | 20.0 | 18.9 |
| 100 | - | 40.8 | 61.7 | 195 | 182 | 100 |
| Standard deviation | 0.2 | 0.2 | 0.2 | 5% | 5% | 5 |
| wwax, wt% | Droplet Diameter, μm |
|---|---|
| 15 | 1.8 ± 0.9 |
| 25 | 2.2 ± 0.9 |
| 35 | 1.9 ± 0.9 |
| 45 | 1.7 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyina, S.O.; Vlasova, A.V.; Gorbunova, I.Y.; Lukashov, N.I.; Kerber, M.L.; Ilyin, S.O. Epoxy Phase-Change Materials Based on Paraffin Wax Stabilized by Asphaltenes. Polymers 2023, 15, 3243. https://doi.org/10.3390/polym15153243
Ilyina SO, Vlasova AV, Gorbunova IY, Lukashov NI, Kerber ML, Ilyin SO. Epoxy Phase-Change Materials Based on Paraffin Wax Stabilized by Asphaltenes. Polymers. 2023; 15(15):3243. https://doi.org/10.3390/polym15153243
Chicago/Turabian StyleIlyina, Svetlana O., Anna V. Vlasova, Irina Y. Gorbunova, Nikolai I. Lukashov, Michael L. Kerber, and Sergey O. Ilyin. 2023. "Epoxy Phase-Change Materials Based on Paraffin Wax Stabilized by Asphaltenes" Polymers 15, no. 15: 3243. https://doi.org/10.3390/polym15153243