Development and Evaluation of Cross-Linked Alginate–Chitosan–Abscisic Acid Blend Gel
Abstract
:1. Introduction
2. Materials and Methods
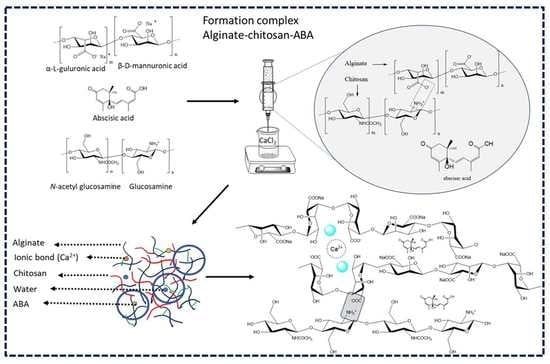
2.1. Building the Molecular Structures of the Polymers
2.2. Molecular Dynamics Simulations of Alginate/Chitosan with and without ABA
2.3. Preparation of the Alginate–Chitosan–ABA Complex with Calcium Cross-Linking
2.4. Preparation of Samples and Thermogravimetric Analysis
2.5. Attenuated Total Reflection–Fourier Transform Infrared (ATR–FTIR) Spectroscopy
2.6. Determination of Abscisic Acid (ABA)
3. Results and Discussion
3.1. In Silico Nanoparticle Formation
3.2. Characterization of Complex, Hydration, and Size
3.3. Thermogravimetric Analysis (TGA) and (DTG) Curves of the Complex Formed in This Study
3.4. Characterization of Complex by Attenuated Total Reflection–Fourier Transform Infrared (ATR–FTIR) Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Eltaweil, A.S.; Abd El-Monaem, E.M.; Elshishini, H.M.; El-Aqapa, H.G.; Hosny, M.; Abdelfatah, A.M.; Ahmed, M.S.; Hammad, E.N.; El-Subruiti, G.M.; Fawzy, M. Recent Developments in Alginate-Based Adsorbents for Removing Phosphate Ions from Wastewater: A Review. RSC Adv. 2022, 12, 8228–8248. [Google Scholar] [CrossRef] [PubMed]
- Yuliana, M.; Ismadji, S.; Lie, J.; Santoso, S.P.; Soetaredjo, F.E.; Waworuntu, G.; Putro, J.N.; Wijaya, C.J. Low-Cost Structured Alginate-Immobilized Bentonite Beads Designed for an Effective Removal of Persistent Antibiotics from Aqueous Solution. Environ. Res. 2022, 207, 112162. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Aisyah, H.A.; Nordin, A.H.; Ngadi, N.; Zuhri, M.Y.M.; Asyraf, M.R.M.; Sapuan, S.M.; Zainudin, E.S.; Sharma, S.; Abral, H. Natural-Fiber-Reinforced Chitosan, Chitosan Blends and Their Nanocomposites for Various Advanced Applications. Polymers 2022, 14, 874. [Google Scholar] [CrossRef] [PubMed]
- Maleki, G.; Woltering, E.J.; Mozafari, M.R. Applications of Chitosan-Based Carrier as an Encapsulating Agent in Food Industry. Trends Food Sci. Technol. 2022, 120, 88–99. [Google Scholar] [CrossRef]
- Albrecht, M.T.; Schiller, N.L. Alginate Lyase (AlgL) Activity Is Required for Alginate Biosynthesis in Pseudomonas Aeruginosa. J. Bacteriol. 2005, 187, 3869–3872. [Google Scholar] [CrossRef] [Green Version]
- In Lee, S.; Choi, S.H.; Lee, E.Y.; Kim, H.S. Molecular cloning, purification, and characterization of a novel polyMG-specific alginate lyase responsible for alginate MG block degradation in Stenotrophomas maltophilia KJ-2. Appl. Microbiol. Biotechnol. 2012, 95, 1643–1653. [Google Scholar] [CrossRef]
- Castro, R.I.; Morales-Quintana, L.; Alvarado, N.; Guzmán, L.; Forero-Doria, O.; Valenzuela-Riffo, F.; Laurie, V.F. Design and Optimization of a Self-Assembling Complex Based on Microencapsulated Calcium Alginate and Glutathione (CAG) Using Response Surface Methodology. Polymers 2021, 13, 2080. [Google Scholar] [CrossRef]
- Castro, R.I.; Laurie, V.F.; Padilla, C.; Carrasco-Sánchez, V. Removal of Ochratoxin A from Red Wine Using Alginate-PVA-L. Plantarum (APLP) Complexes: A Preliminary Study. Toxins 2022, 14, 230. [Google Scholar] [CrossRef]
- Lillo, L.E.; Matsuhiro, B. Chemical Modifications of 1→4-2-Amino-2-Deoxy-α-d-Galactan. Carbohydr. Polym. 2003, 51, 317–325. [Google Scholar] [CrossRef]
- Yáñez, O.; Alegría-Arcos, M.; Suardiaz, R.; Morales-Quintana, L.; Castro, R.I.; Palma-Olate, J.; Galarza, C.; Catagua-González, Á.; Rojas-Pérez, V.; Urra, G.; et al. Calcium-Alginate-Chitosan Nanoparticle as a Potential Solution for Pesticide Removal, a Computational Approach. Polymers 2023, 15, 3020. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent Advances of Chitosan Nanoparticles as Drug Carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar]
- Wang, G.; Li, R.; Parseh, B.; Du, G. Prospects and Challenges of Anticancer Agents’ Delivery via Chitosan-Based Drug Carriers to Combat Breast Cancer: A Review. Carbohydr. Polym. 2021, 268, 118192. [Google Scholar] [CrossRef]
- Gómez Chabala, L.F.; Cuartas, C.E.E.; López, M.E.L. Release Behavior and Antibacterial Activity of Chitosan/Alginate Blends with Aloe Vera and Silver Nanoparticles. Mar. Drugs 2017, 15, 328. [Google Scholar] [CrossRef] [Green Version]
- Yongmei, X.; Changyou, Z.; Lihong, F.; Le, W.; Hua, Z. Preparation of Dual Crosslinked Alginate–Chitosan Blends Gel Beads and in Vitro Controlled Release in Oral Site-Specific Drug Delivery System. Int. J. Pharm. 2007, 336, 329–337. [Google Scholar]
- Bai, Y.; Wu, W. The Neutral Protease Immobilization: Physical Characterization of Sodium Alginate-Chitosan Gel Beads. Appl. Biochem. Biotechnol. 2022, 194, 2269–2283. [Google Scholar] [CrossRef]
- Gotoh, T.; Matsushima, K.; Kikuchi, K.-I. Preparation of Alginate–Chitosan Hybrid Gel Beads and Adsorption of Divalent Metal Ions. Chemosphere 2004, 55, 135–140. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Hussein, H.S.; Ragab, A.H.; AlMasoud, N.; Ghfar, A.A. Investigation the Effects of Green-Synthesized Copper Nanoparticles on the Performance of Activated Carbon-Chitosan-Alginate for the Removal of Cr (VI) from Aqueous Solution. Molecules 2021, 26, 2617. [Google Scholar] [CrossRef]
- Liao, P.; Dai, S.; Lian, Z.; Tong, X.; Yang, S.; Chen, Y.; Qi, W.; Peng, X.; Wang, H.; Jiang, L. The Layered Encapsulation of Vitamin B2 and β-Carotene in Multilayer Alginate/Chitosan Gel Microspheres: Improving the Bioaccessibility of Vitamin B2 and β-Carotene. Foods 2022, 11, 20. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Ghaffar, A.; Gull, N.; Azam, M.; Mehmood, A.; Ghauri, A.A.; Khan, R.U. Novel PH-Responsive Chitosan/Sodium Alginate/PEG Based Hydrogels for Release of Sodium Ceftriaxone. Mater. Chem. Phys. 2022, 277, 125456. [Google Scholar] [CrossRef]
- Li, D.; Li, L.; Luo, Z.; Mou, W.; Mao, L.; Ying, T. Comparative transcriptome analysis reveals the influence of abscisic acid on the metabolism of pigments, ascorbic acid and folic acid during strawberry fruit ripening. PLoS ONE 2015, 10, e0130037. [Google Scholar] [CrossRef]
- Gu, T.; Jia, S.; Huang, X.; Wang, L.; Fu, W.; Huo, G.; Ding, J.; Li, Y. Transcriptome and hormone analyses provide insights into hormonal regulation in strawberry ripening. Planta 2019, 250, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.I.; Gonzalez-Feliu, A.; Valenzuela-Riffo, F.; Parra-Palma, C.; Morales-Quintana, L. Changes in the cell wall components produced by exogenous abscisic acid treatment in strawberry fruit. Cellulose 2021, 28, 1555–1570. [Google Scholar] [CrossRef]
- Chen, J.; Mao, L.; Lu, W.; Ying, T.; Luo, Z. Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid. Planta 2016, 243, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Parwez, R.; Aftab, T.; Gill, S.S.; Naeem, M. Abscisic Acid Signaling and Crosstalk with Phytohormones in Regulation of Environmental Stress Responses. Environ. Exp. Bot. 2022, 199, 104885. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of Seed Germination and Abiotic Stresses by Gibberellins and Abscisic Acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [Green Version]
- Bustos, D.; Hernández-Rodríguez, E.W.; Castro, R.I.; Morales-Quintana, L. Structural Effects of PH Variation and Calcium Amount on the Microencapsulation of Glutathione in Alginate Polymers. Biomed Res. Int. 2022, 2022, 5576090. [Google Scholar] [CrossRef]
- Valdés, C.; Valdés, O.; Bustos, D.; Abril, D.; Cabrera-Barjas, G.; Pereira, A.; Villaseñor, J.; Polo-Cuadrado, E.; Carreño, G.; Durán-Lara, E.F. Use of Poly (Vinyl Alcohol)-Malic Acid (CLHPMA) Hydrogels and Chitosan Coated Calcium Alginate (CCCA) Microparticles as Potential Sorbent Phases for the Extraction and Quantitative Determination of Pesticides from Aqueous Solutions. Polymers 2021, 13, 3993. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Maestro, S. Schrödinger Release 2021-1; Schrödinger LLC: New York, NY, USA, 2020. [Google Scholar]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [Optimized Potentials for Liquid Simulations] Potential Functions for Proteins, Energy Minimizations for Crystals of Cyclic Peptides and Crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Qi, B.; Wu, C.; Liang, H.; Cui, K.; Fahad, S.; Wang, M.; Liu, B.; Nie, L.; Huang, J.; Tang, H. Optimized High-Performance Liquid Chromatography Method for Determining Nine Cytokinins, Indole-3-Acetic Acid and Abscisic Acid. Sustainability 2021, 13, 6998. [Google Scholar] [CrossRef]
- Avila-Salas, F.; Rodriguez Nuñez, Y.A.; Marican, A.; Castro, R.I.; Villaseñor, J.; Santos, L.S.; Wehinger, S.; Durán-Lara, E.F. Rational Development of a Novel Hydrogel as a PH-Sensitive Controlled Release System for Nifedipine. Polymers 2018, 10, 806. [Google Scholar] [CrossRef] [Green Version]
- Plazinski, W. Molecular Basis of Calcium Binding by Polyguluronate Chains. Revising the Egg-box Model. J. Comput. Chem. 2011, 32, 2988–2995. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Król, Ż.; Oziembłowski, M.; Jarmoluk, A. Effect of Film-Forming Alginate/Chitosan Polyelectrolyte Complex on the Storage Quality of Pork. Molecules 2017, 22, 98. [Google Scholar] [CrossRef] [Green Version]
- Nepomuceno, N.C.; Fook, M.V.L.; Ries, A.; Mija, A.; Wellen, R.M.R. Bio-Based Epoxy Resins of Epoxidized Soybean Oil Cured with Salicylic Acid Loaded with Chitosan: Evaluation of Physical–Chemical Properties. J. Polym. Environ. 2023, 31, 2566–2575. [Google Scholar] [CrossRef]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit Ripening Phenomena–an Overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Holme, H.K.; Davidsen, L.; Kristiansen, A.; Smidsrød, O. Kinetics and Mechanisms of Depolymerization of Alginate and Chitosan in Aqueous Solution. Carbohydr. Polym. 2008, 73, 656–664. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, C. Fire-Retardant Multilayer Assembled on Polyester Fabric from Water-Soluble Chitosan, Sodium Alginate and Divalent Metal Ion. Int. J. Biol. Macromol. 2018, 119, 1083–1089. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Khutoryanskiy, V.V.; Charalampopoulos, D. Layer-by-Layer Coating of Alginate Matrices with Chitosan–Alginate for the Improved Survival and Targeted Delivery of Probiotic Bacteria after Oral Administration. J. Mater. Chem. B 2013, 1, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Mndlovu, H.; du Toit, L.C.; Kumar, P.; Marimuthu, T.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Development of a Fluid-Absorptive Alginate-Chitosan Bioplatform for Potential Application as a Wound Dressing. Carbohydr. Polym. 2019, 222, 114988. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Donoso, W.; Castro, R.I.; Guzmán, L.; López-Cabaña, Z.; Nachtigall, F.M.; Santos, L.S. Fast Detection of Listeria Monocytogenes through a Nanohybrid Quantum Dot Complex. Anal. Bioanal. Chem. 2017, 409, 5359–5371. [Google Scholar] [CrossRef] [PubMed]







| Sample | Complex + ABA | ||
|---|---|---|---|
| Size mm (n = 3) | 0.320 ± 0.02 | 0.345 ± 0.04 | 0.410 ± 0.04 |
| Water abs (%) (region 2) | 77.22 | 76.44 | 90.12 |
| Total water (%) | 88.61 | 88.22 | 95.06 |
| Ratio: alginate:chitosan | 2:1 | 1:1 | 1:2 |
| Concentration of ABA in the complex per 0.9 mg complex | 0.038 | 0.023 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustos, D.; Guzmán, L.; Valdés, O.; Muñoz-Vera, M.; Morales-Quintana, L.; Castro, R.I. Development and Evaluation of Cross-Linked Alginate–Chitosan–Abscisic Acid Blend Gel. Polymers 2023, 15, 3217. https://doi.org/10.3390/polym15153217
Bustos D, Guzmán L, Valdés O, Muñoz-Vera M, Morales-Quintana L, Castro RI. Development and Evaluation of Cross-Linked Alginate–Chitosan–Abscisic Acid Blend Gel. Polymers. 2023; 15(15):3217. https://doi.org/10.3390/polym15153217
Chicago/Turabian StyleBustos, Daniel, Luis Guzmán, Oscar Valdés, Marcelo Muñoz-Vera, Luis Morales-Quintana, and Ricardo I. Castro. 2023. "Development and Evaluation of Cross-Linked Alginate–Chitosan–Abscisic Acid Blend Gel" Polymers 15, no. 15: 3217. https://doi.org/10.3390/polym15153217